Immunological Properties of Neural Crest Cells Derived from Human Induced Pluripotent Stem Cells
- PMID: 30251915
- PMCID: PMC6350061
- DOI: 10.1089/scd.2018.0058
Immunological Properties of Neural Crest Cells Derived from Human Induced Pluripotent Stem Cells
Abstract
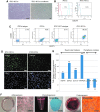
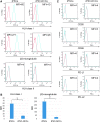
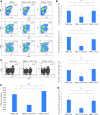
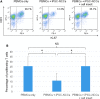
Collecting sufficient quantities of primary neural crest cells (NCCs) for experiments is difficult, as NCCs are embryonic transient tissue that basically does not proliferate. We successfully induced NCCs from human induced pluripotent stem cells (iPSCs) in accordance with a previously described method with some modifications. The protocol used in this study efficiently produced large amounts of iPSC-derived NCCs (iPSC-NCCs). Many researchers have recently produced large amounts of iPSC-NCCs and used these to examine the physiological properties, such as migratory activity, and the potential for medical uses such as wound healing. Immunological properties of NCCs are yet to be reported. Therefore, the purpose of this study was to assess the immunological properties of human iPSC-NCCs. Our current study showed that iPSC-NCCs were hypoimmunogenic and had immunosuppressive properties in vitro. Expression of HLA class I molecules on iPSC-NCCs was lower than that observed for iPSCs, and there was no expression of HLA class II and costimulatory molecules on the cells. With regard to the immunosuppressive properties, iPSC-NCCs greatly inhibited T cell activation (cell proliferation and production of inflammatory cytokines) after stimulation. iPSC-NCCs constitutively expressed membrane-bound TGF-β, and TGF-β produced by iPSC-NCCs played a critical role in T cell suppression. Thus, cultured human NCCs can fully suppress T cell activation in vitro. This study may contribute to the realization of using stem cell-derived NCCs in cell-based medicine.
Keywords: T cells; eye; immunosuppression; neural crest cells.
Conflict of interest statement
No competing financial interests exist.
Figures


Similar articles
-
Capacity of Retinal Ganglion Cells Derived from Human Induced Pluripotent Stem Cells to Suppress T-Cells.Int J Mol Sci. 2020 Oct 22;21(21):7831. doi: 10.3390/ijms21217831. Int J Mol Sci. 2020. PMID: 33105725 Free PMC article.
-
CHARGE syndrome modeling using patient-iPSCs reveals defective migration of neural crest cells harboring CHD7 mutations.Elife. 2017 Nov 28;6:e21114. doi: 10.7554/eLife.21114. Elife. 2017. PMID: 29179815 Free PMC article.
-
The stemness of neural crest cells and their derivatives.Birth Defects Res C Embryo Today. 2014 Sep;102(3):251-62. doi: 10.1002/bdrc.21079. Epub 2014 Sep 15. Birth Defects Res C Embryo Today. 2014. PMID: 25219876 Review.
-
Generation of Neural Crest-Like Cells From Human Periodontal Ligament Cell-Derived Induced Pluripotent Stem Cells.J Cell Physiol. 2017 Feb;232(2):402-416. doi: 10.1002/jcp.25437. Epub 2016 Jun 2. J Cell Physiol. 2017. PMID: 27206577
-
Modeling human congenital disorders with neural crest developmental defects using patient-derived induced pluripotent stem cells.Regen Ther. 2021 Aug 24;18:275-280. doi: 10.1016/j.reth.2021.08.001. eCollection 2021 Dec. Regen Ther. 2021. PMID: 34504908 Free PMC article. Review.
Cited by
-
Neural crest-like stem cells for tissue regeneration.Stem Cells Transl Med. 2021 May;10(5):681-693. doi: 10.1002/sctm.20-0361. Epub 2021 Feb 2. Stem Cells Transl Med. 2021. PMID: 33533168 Free PMC article. Review.
-
Potential application of human neural crest-derived nasal turbinate stem cells for the treatment of neuropathology and impaired cognition in models of Alzheimer's disease.Stem Cell Res Ther. 2021 Jul 13;12(1):402. doi: 10.1186/s13287-021-02489-1. Stem Cell Res Ther. 2021. PMID: 34256823 Free PMC article.
-
Capacity of Retinal Ganglion Cells Derived from Human Induced Pluripotent Stem Cells to Suppress T-Cells.Int J Mol Sci. 2020 Oct 22;21(21):7831. doi: 10.3390/ijms21217831. Int J Mol Sci. 2020. PMID: 33105725 Free PMC article.
-
Role of Maternal Infections and Inflammatory Responses on Craniofacial Development.Front Oral Health. 2021 Sep 6;2:735634. doi: 10.3389/froh.2021.735634. eCollection 2021. Front Oral Health. 2021. PMID: 35048051 Free PMC article. Review.
-
Innate and Adaptive Immunity Linked to Recognition of Antigens Shared by Neural Crest-Derived Tumors.Cancers (Basel). 2020 Mar 31;12(4):840. doi: 10.3390/cancers12040840. Cancers (Basel). 2020. PMID: 32244473 Free PMC article. Review.
References
-
- Reneker LW, Silversides DW, Xu L. and Overbeek PA. (2000). Formation of corneal endothelium is essential for anterior segment development - a transgenic mouse model of anterior segment dysgenesis. Development 127:533–542 - PubMed
-
- Fournier BP, Loison-Robert LS, Ferré FC, Owen GR, Larjava H. and Häkkinen L. (2016). Characterisation of human gingival neural crest-derived stem cells in monolayer and neurosphere cultures. Eur Cells Mater 31:40–58 - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
