Sodium propionate and sodium butyrate effects on histone deacetylase (HDAC) activity, histone acetylation, and inflammatory gene expression in bovine mammary epithelial cells
- PMID: 30252114
- PMCID: PMC6276571
- DOI: 10.1093/jas/sky373
Sodium propionate and sodium butyrate effects on histone deacetylase (HDAC) activity, histone acetylation, and inflammatory gene expression in bovine mammary epithelial cells
Abstract
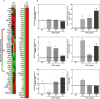
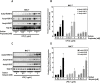
Histone deacetylase (HDAC) inhibition attenuates inflammation in rodents and short-chain fatty acids (SCFAs) are effective HDAC inhibitors. Therefore, the objective of this study was to evaluate the role of the SCFAs sodium propionate (SP) and sodium butyrate (SB) as HDAC-dependent regulators of inflammatory gene expression in bovine mammary epithelial cells (MAC-Ts). We postulated that SP and SB would decrease inflammation in MAC-Ts by inhibiting HDAC activity and increasing histone H3 acetylation and consequently decreasing inflammatory gene expression. For this study, MAC-Ts stimulated with lipopolysaccharide (LPS) were used as a model for bovine mammary epithelial cell inflammation. MAC-Ts were cultured in a basal medium. Cell lysates were incubated with SP or SB (0 to 5 mM) for 2 h prior to HDAC substrates incubation for an additional 2 h and HDACs activity was determined. Next, cells were pretreated with SP or SB (0 to 3.0 mM) for 2 h prior to LPS (1 µg/mL) stimulation for an additional 2 h and assessed for histone H3 acetylation. Then, cells were pretreated with SP or SB (1 mM) for 24 h prior to LPS (1 µg/mL) stimulation for an additional 2 h and RNA was isolated for inflammatory gene expression evaluation by PCR array and gene validation was performed using quantitative real-time PCR. One-way ANOVA followed by Tukey post hoc analysis was conducted and statistical significance set at P < 0.05. SP and SB concentration-dependently and selectively inhibited class I HDAC activity, which differed between SCFAs, where SB inhibited (P < 0.05) HDACs 2, 3, and 8, while SP inhibited (P < 0.05) HDACs 2 and 8. Histone H3 acetylation was concentration-dependently increased by SCFAs and likewise the differential regulation of HDAC activity, SCFAs effected differently histone H3 acetylation, where SB increased (P < 0.05) H3K9/14, H3K18 and H3K27 acetylation, while SP increased (P < 0.05) H3K9/14 and H3K18 acetylation. However, SCFAs did not decrease (P > 0.05) overall inflammatory gene expression. Under our experimental conditions, findings suggest that in MAC-Ts, SCFAs regulate epigenetic markers on nucleosomal DNA in addition to regulation of inflammatory gene events independent of HDAC activity. Nevertheless, examination of SCFAs and/or HDACs inhibitors in bovine mammary gland is worth being further investigated to delineate the potential impact of HDAC inhibition and histones hyperacetylation on mammary gland tissue inflammation.
Figures

Similar articles
-
Bacterial endotoxin decreased histone H3 acetylation of bovine mammary epithelial cells and the adverse effect was suppressed by sodium butyrate.BMC Vet Res. 2019 Jul 29;15(1):267. doi: 10.1186/s12917-019-2007-5. BMC Vet Res. 2019. PMID: 31357995 Free PMC article.
-
Sodium butyrate reduces bovine mammary epithelial cell inflammatory responses induced by exogenous lipopolysaccharide, by inactivating NF-κB signaling.J Dairy Sci. 2020 Sep;103(9):8388-8397. doi: 10.3168/jds.2020-18189. Epub 2020 Jul 1. J Dairy Sci. 2020. PMID: 32622605
-
Sodium butyrate promotes lipopolysaccharide-induced innate immune responses by enhancing mitogen-activated protein kinase activation and histone acetylation in bovine mammary epithelial cells.J Dairy Sci. 2020 Dec;103(12):11636-11652. doi: 10.3168/jds.2020-18198. Epub 2020 Oct 1. J Dairy Sci. 2020. PMID: 33010913
-
[Epigenetic mechanisms and alcohol use disorders: a potential therapeutic target].Biol Aujourdhui. 2017;211(1):83-91. doi: 10.1051/jbio/2017014. Epub 2017 Jul 6. Biol Aujourdhui. 2017. PMID: 28682229 Review. French.
-
Short-chain fatty acid inhibitors of histone deacetylases: promising anticancer therapeutics?Curr Cancer Drug Targets. 2003 Jun;3(3):219-36. doi: 10.2174/1568009033481994. Curr Cancer Drug Targets. 2003. PMID: 12769690 Review.
Cited by
-
The JMJD3 histone demethylase inhibitor GSK-J1 ameliorates lipopolysaccharide-induced inflammation in a mastitis model.J Biol Chem. 2022 Jun;298(6):102017. doi: 10.1016/j.jbc.2022.102017. Epub 2022 May 6. J Biol Chem. 2022. PMID: 35526564 Free PMC article.
-
Sodium acetate regulates milk fat synthesis through the activation of GPR41/GPR43 signaling pathway.Front Nutr. 2023 Feb 16;10:1098715. doi: 10.3389/fnut.2023.1098715. eCollection 2023. Front Nutr. 2023. PMID: 36969813 Free PMC article.
-
Short-chain fatty acids as modulators of redox signaling in health and disease.Redox Biol. 2021 Nov;47:102165. doi: 10.1016/j.redox.2021.102165. Epub 2021 Oct 14. Redox Biol. 2021. PMID: 34662811 Free PMC article. Review.
-
Targeting DNA Methylation in the Adult Brain through Diet.Nutrients. 2021 Nov 8;13(11):3979. doi: 10.3390/nu13113979. Nutrients. 2021. PMID: 34836233 Free PMC article. Review.
-
Gut microbial metabolite targets HDAC3-FOXK1-interferon axis in fibroblast-like synoviocytes to ameliorate rheumatoid arthritis.Bone Res. 2024 May 23;12(1):31. doi: 10.1038/s41413-024-00336-6. Bone Res. 2024. PMID: 38782893 Free PMC article.
References
-
- Alva-Murillo N., Ochoa-Zarzosa A., and López-Meza J. E.. 2012. Short chain fatty acids (propionic and hexanoic) decrease Staphylococcus aureus internalization into bovine mammary epithelial cells and modulate antimicrobial peptide expression. Vet. Microbiol. 155:324–331. doi:10.1016/j.vetmic.2011.08.025 - DOI - PubMed
-
- Angiolilli C., Kabala P. A., Grabiec A. M., Van Baarsen I. M., Ferguson B. S., García S., Malvar Fernandez B., McKinsey T. A., Tak P. P., Fossati G.,. et al. 2017. Histone deacetylase 3 regulates the inflammatory gene expression programme of rheumatoid arthritis fibroblast-like synoviocytes. Ann. Rheum. Dis. 76:277–285. doi:10.1136/annrheumdis-2015-209064 - DOI - PMC - PubMed
-
- Baumann H., and Gauldie J.. 1994. The acute phase response. Immunol. Today 15:74. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

