A single synonymous mutation determines the phosphorylation and stability of the nascent protein
- PMID: 30252118
- PMCID: PMC6734142
- DOI: 10.1093/jmcb/mjy049
A single synonymous mutation determines the phosphorylation and stability of the nascent protein
Abstract
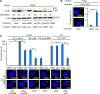
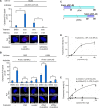
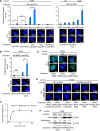
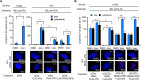
p53 is an intrinsically disordered protein with a large number of post-translational modifications and interacting partners. The hierarchical order and subcellular location of these events are still poorly understood. The activation of p53 during the DNA damage response (DDR) requires a switch in the activity of the E3 ubiquitin ligase MDM2 from a negative to a positive regulator of p53. This is mediated by the ATM kinase that regulates the binding of MDM2 to the p53 mRNA facilitating an increase in p53 synthesis. Here we show that the binding of MDM2 to the p53 mRNA brings ATM to the p53 polysome where it phosphorylates the nascent p53 at serine 15 and prevents MDM2-mediated degradation of p53. A single synonymous mutation in p53 codon 22 (L22L) prevents the phosphorylation of the nascent p53 protein and the stabilization of p53 following genotoxic stress. The ATM trafficking from the nucleus to the p53 polysome is mediated by MDM2, which requires its interaction with the ribosomal proteins RPL5 and RPL11. These results show how the ATM kinase phosphorylates the p53 protein while it is being synthesized and offer a novel mechanism whereby a single synonymous mutation controls the stability and activity of the encoded protein.
Keywords: ATM kinase; MDM2; cell signaling; intrinsically disordered proteins; p53 messenger RNA; synonymous mutations.
© The Author(s) (2018). Published by Oxford University Press on behalf of Journal of Molecular Cell Biology, IBCB, SIBS, CAS. All rights reserved.
Figures

Similar articles
-
The p53 mRNA-Mdm2 interaction controls Mdm2 nuclear trafficking and is required for p53 activation following DNA damage.Cancer Cell. 2012 Jan 17;21(1):25-35. doi: 10.1016/j.ccr.2011.11.016. Cancer Cell. 2012. PMID: 22264786
-
ATM and Chk2-dependent phosphorylation of MDMX contribute to p53 activation after DNA damage.EMBO J. 2005 Oct 5;24(19):3411-22. doi: 10.1038/sj.emboj.7600812. Epub 2005 Sep 15. EMBO J. 2005. PMID: 16163388 Free PMC article.
-
Phosphorylation of Daxx by ATM contributes to DNA damage-induced p53 activation.PLoS One. 2013;8(2):e55813. doi: 10.1371/journal.pone.0055813. Epub 2013 Feb 6. PLoS One. 2013. PMID: 23405218 Free PMC article.
-
ATM-mediated phosphorylations inhibit Mdmx/Mdm2 stabilization by HAUSP in favor of p53 activation.Cell Cycle. 2005 Sep;4(9):1166-70. doi: 10.4161/cc.4.9.1981. Epub 2005 Sep 29. Cell Cycle. 2005. PMID: 16082221 Review.
-
Mdm2 links genotoxic stress and metabolism to p53.Protein Cell. 2010 Dec;1(12):1063-72. doi: 10.1007/s13238-010-0140-9. Epub 2011 Jan 8. Protein Cell. 2010. PMID: 21213101 Free PMC article. Review.
Cited by
-
Synonymous Variants: Necessary Nuance in Our Understanding of Cancer Drivers and Treatment Outcomes.J Natl Cancer Inst. 2022 Aug 8;114(8):1072-1094. doi: 10.1093/jnci/djac090. J Natl Cancer Inst. 2022. PMID: 35477782 Free PMC article. Review.
-
MDM2's dual mRNA binding domains co-ordinate its oncogenic and tumour suppressor activities.Nucleic Acids Res. 2020 Jul 9;48(12):6775-6787. doi: 10.1093/nar/gkaa431. Nucleic Acids Res. 2020. PMID: 32453417 Free PMC article.
-
The Elephant Evolved p53 Isoforms that Escape MDM2-Mediated Repression and Cancer.Mol Biol Evol. 2022 Jul 2;39(7):msac149. doi: 10.1093/molbev/msac149. Mol Biol Evol. 2022. PMID: 35792674 Free PMC article.
-
A pan-cancer analysis of synonymous mutations.Nat Commun. 2019 Jun 12;10(1):2569. doi: 10.1038/s41467-019-10489-2. Nat Commun. 2019. PMID: 31189880 Free PMC article.
-
p53 mRNA Metabolism Links with the DNA Damage Response.Genes (Basel). 2021 Sep 20;12(9):1446. doi: 10.3390/genes12091446. Genes (Basel). 2021. PMID: 34573428 Free PMC article. Review.
References
-
- Banin S., Moyal L., Shieh S., et al. . (1998). Enhanced phosphorylation of p53 by ATM in response to DNA damage. Science 281, 1674–1677. - PubMed
-
- Bursac S., Brdovcak M.C., Donati G., et al. . (2014). Activation of the tumor suppressor p53 upon impairment of ribosome biogenesis. Biochim. Biophys. Acta 1842, 817–830. - PubMed
-
- Candeias M.M., Malbert-Colas L., Powell D.J., et al. . (2008). P53 mRNA controls p53 activity by managing Mdm2 functions. Nat. Cell Biol. 10, 1098–1105. - PubMed
-
- Canman C.E., Lim D.S., Cimprich K.A., et al. . (1998). Activation of the ATM kinase by ionizing radiation and phosphorylation of p53. Science 281, 1677–1679. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

