Input-output organization of the mouse claustrum
- PMID: 30252130
- PMCID: PMC6196111
- DOI: 10.1002/cne.24502
Input-output organization of the mouse claustrum
Abstract
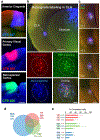
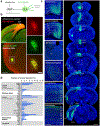
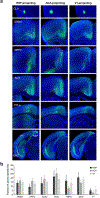
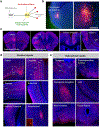
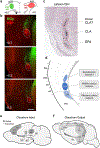
Progress in determining the precise organization and function of the claustrum (CLA) has been hindered by the difficulty in reliably targeting these neurons. To overcome this, we used a projection-based targeting strategy to selectively label CLA principal neurons. Combined with adeno-associated virus (AAV) and monosynaptic rabies tracing techniques, we systematically examined the pre-synaptic input and axonal output of this structure. We found that CLA neurons projecting to retrosplenial cortex (RSP) collateralize extensively to innervate a variety of higher-order cortical regions. No subcortical labeling was found, with the exception of sparse terminals in the basolateral amygdala (BLA). This pattern of output was similar to cingulate- and visual cortex-projecting CLA neurons, suggesting a common targeting scheme among these projection-defined populations. Rabies virus tracing directly demonstrated widespread synaptic inputs to RSP-projecting CLA neurons from both cortical and subcortical areas. The strongest inputs arose from classically defined limbic regions, including medial prefrontal cortex, anterior cingulate, BLA, ventral hippocampus, and neuromodulatory systems such as the dorsal raphe and cholinergic basal forebrain. These results suggest that the CLA may integrate information related to the emotional salience of stimuli and may globally modulate cortical state by broadcasting its output uniformly across a variety of higher cognitive centers.
Keywords: AAV; RRID:AB_141844; RRID:AB_2079751; RRID:AB_2572212; claustro-cortical network; claustrum; connectivity; frontal cortex; limbic; neuromodulatory; rabies; retrograde tracing.
© 2018 Wiley Periodicals, Inc.
Figures

References
-
- Brown Solange P; Brian Mathur; Olsen Shawn R; Pierre-Herve Luppi; Bickford Martha E; Citri A. (2017). New Breakthroughs in Understanding the Role of Functional Interactions between the Neocortex and the Claustrum. The Journal of Neuroscience, 37(45), 10877–10881. http://doi.org/DOI:10.1523/JNEUROSCI.1837-17.2017 - DOI - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

