RARβ acts as both an upstream regulator and downstream effector of miR-22, which epigenetically regulates NUR77 to induce apoptosis of colon cancer cells
- PMID: 30252536
- PMCID: PMC6338632
- DOI: 10.1096/fj.201801390R
RARβ acts as both an upstream regulator and downstream effector of miR-22, which epigenetically regulates NUR77 to induce apoptosis of colon cancer cells
Abstract
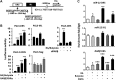
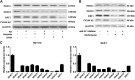
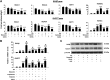
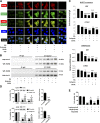
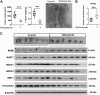
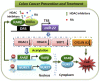
This study investigates the mechanism and consequences of microRNA-22 ( miR-22) induction. Our data revealed for the first time that retinoic acid (RA) and histone deacetylase (HDAC) inhibitors, including short-chain fatty acids and suberanilohydroxamic acid (SAHA), could individually or in combination induce miR-22. This induction was mediated via RA receptor β (RARβ) binding to a direct repeat 5 (DR5) motif. In addition, we uncovered HDAC1 as a novel miR-22 target. In an miR-22-dependent manner, HDAC inhibitors and RA reduced HDAC1, HDAC4, and sirtuin 1 (SIRT1), which were involved in chromatin remodeling of the RARβ and nerve growth factor IB ( NUR77). Thus, HDAC inhibitors and RA-induced miR-22 resulted in simultaneous induction of cytoplasmic RARβ and NUR77, leading to apoptosis of colon cancer cells. In mice, miR-22 and its inducers inhibited the growth of xenograft colon cancer. Moreover, tumor size reduction was accompanied by elevated miR-22, NUR77, and RARβ and by reduced HDACs. In human colon polyps and adenocarcinomas, miR-22 and RARβ were consistently reduced, which was associated with elevated HDAC1, HDAC4, and SIRT1 in colon adenocarcinomas. Results from this study revealed a novel anticancer mechanism of RARβ via miR-22 induction to epigenetically regulate itself and NUR77, providing a promising cancer treatment modality using miR-22 and its inducers.-Hu, Y., French, S. W., Chau, T., Liu, H.-X., Sheng, L., Wei, F., Stondell, J., Garcia, J. C., Du, Y., Bowlus, C. L., Wan, Y.-J. Y. RARβ acts as both an upstream regulator and downstream effector of miR-22, which epigenetically regulates NUR77 to induce apoptosis of colon cancer cells.
Keywords: butyrate; nuclear receptor; propionate; protein deacetylase; short-chain fatty acid.
Conflict of interest statement
The authors thank Dr. Prasant Kumar Jena and Nidhi Nagar (University of California, Davis Health System) for their assistance in preparation of this manuscript. This study was supported by U.S. National Institutes of Health (NIH) National Cancer Institute Grant R01CA222490 (to Y.-J.Y.W.); a Science Translation and Innovative Research (STAIR) Grant (to Y.-J.Y.W.); and National Natural Science Foundation of China Grant 81772572 (to Y.D.). The authors declare no conflicts of interest.
Figures




References
-
- Tsuchiya, N., Izumiya, M., Ogata-Kawata, H., Okamoto, K., Fujiwara, Y., Nakai, M., Okabe, A., Schetter, A. J., Bowman, E. D., Midorikawa, Y., Sugiyama, Y., Aburatani, H., Harris, C. C., Nakagama, H. (2011) Tumor suppressor miR-22 determines p53-dependent cellular fate through post-transcriptional regulation of p21. Cancer Res. 71, 4628–4639 - PMC - PubMed
-
- Xiong, J., Du, Q., Liang, Z. (2010) Tumor-suppressive microRNA-22 inhibits the transcription of E-box-containing c-Myc target genes by silencing c-Myc binding protein. Oncogene 29, 4980–4988 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

