Immunogenicity and safety of the Haemophilus influenzae type b and Neisseria meningitidis serogroups C and Y-tetanus toxoid conjugate vaccine co-administered with human rotavirus, hepatitis A and 13-valent pneumococcal conjugate vaccines: results from a phase III, randomized, multicenter study in infants
- PMID: 30252603
- PMCID: PMC6422469
- DOI: 10.1080/21645515.2018.1526586
Immunogenicity and safety of the Haemophilus influenzae type b and Neisseria meningitidis serogroups C and Y-tetanus toxoid conjugate vaccine co-administered with human rotavirus, hepatitis A and 13-valent pneumococcal conjugate vaccines: results from a phase III, randomized, multicenter study in infants
Abstract
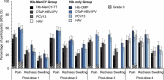
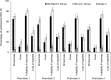
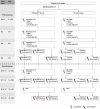
This phase III, open-label, randomized study (NCT01978093) evaluated the immunogenicity and safety of co-administered Haemophilus influenzae type b-Neisseria meningitidis serogroups C and Y-tetanus toxoid conjugate vaccine (Hib-MenCY-TT) with human rotavirus vaccine (HRV), hepatitis A vaccine (HAV) and 13-valent pneumococcal conjugate vaccine (PCV13). We randomized 600 infants (1:1) to receive 4 doses of Hib-MenCY-TT at 2, 4, 6 and 12-15 months of age or 3 doses of Hib vaccine conjugated to N. meningitidis outer membrane protein complex (Hib-OMP) at 2, 4 and 12-15 months of age. All infants received HRV at 2 and 4 months of age, PCV13 at 2, 4, 6 and 12-15 months of age, HAV at 12-15 and 18-21 months of age, and diphtheria-tetanus-acellular pertussis-hepatitis B-inactivated poliovirus vaccine at 2, 4 and 6 months of age. We measured immune responses against HRV, HAV and Hib with enzyme-linked immunosorbent assays, and against MenC/MenY with serum bactericidal assays using human complement. The 4-dose vaccination series with Hib-MenCY-TT induced a robust immune response against Hib, which was non-inferior to that induced by a 3-dose vaccination series with Hib-OMP, and against MenC and MenY. Hib-MenCY-TT did not interfere with immune responses to concomitantly administered HRV, PCV13 and HAV. We did not identify any safety concern. In conclusion, we showed that 4-dose vaccination series with Hib-MenCY-TT during infancy did not interfere with immune responses of co-administered HRV, PCV13 and HAV, induced robust immune responses against Hib, MenC and MenY, and had a clinically acceptable safety profile.
Keywords: serogroups C and Y; type b; co-administration; hepatitis A; human rotavirus; infant; vaccination.
Figures


Similar articles
-
Combined Haemophilus influenzae type b and Neisseria meningitidis serogroup C (HibMenC) or serogroup C and Y-tetanus toxoid conjugate (and HibMenCY) vaccines are well-tolerated and immunogenic when administered according to the 2,3,4 months schedule with a fourth dose at 12-18 months of age.Hum Vaccin. 2010 Aug;6(8):640-51. doi: 10.4161/hv.6.8.12154. Hum Vaccin. 2010. PMID: 20697200 Clinical Trial.
-
Randomized trial to assess immunogenicity and safety of Haemophilus influenzae type b and Neisseria meningitidis serogroups C and Y-tetanus toxoid conjugate vaccine in infants.Pediatr Infect Dis J. 2010 Jan;29(1):48-52. doi: 10.1097/INF.0b013e3181c3ce88. Pediatr Infect Dis J. 2010. PMID: 20035207 Clinical Trial.
-
A combined Haemophilus influenzae type B Neisseria meningitidis serogroup C tetanus toxoid conjugate vaccine is immunogenic and well-tolerated when coadministered with diphtheria, tetanus, acellular pertussis hepatitis B-inactivated poliovirus at 3, 5 and 11 months of age: results of an open, randomized, controlled study.Pediatr Infect Dis J. 2013 May;32(5):521-9. doi: 10.1097/INF.0b013e31827e22e3. Pediatr Infect Dis J. 2013. PMID: 23190785 Clinical Trial.
-
Meningococcal groups C and Y and haemophilus B tetanus toxoid conjugate vaccine (HibMenCY-TT; MenHibrix(®)): a review.Drugs. 2013 May;73(7):703-13. doi: 10.1007/s40265-013-0048-9. Drugs. 2013. PMID: 23649970 Review.
-
MenHibrix: a new combination meningococcal vaccine for infants and toddlers.Ann Pharmacother. 2014 Mar;48(3):404-11. doi: 10.1177/1060028013514375. Epub 2013 Dec 18. Ann Pharmacother. 2014. PMID: 24353263 Review.
Cited by
-
Co-administration of vaccines: a focus on tetravalent Measles-Mumps-Rubella-Varicella (MMRV) and meningococcal C conjugate vaccines.Hum Vaccin Immunother. 2020 Jun 2;16(6):1313-1321. doi: 10.1080/21645515.2019.1688032. Epub 2019 Dec 10. Hum Vaccin Immunother. 2020. PMID: 31810408 Free PMC article.
-
A Novel High-Potency Tetanus Vaccine.mBio. 2020 Aug 11;11(4):e01668-20. doi: 10.1128/mBio.01668-20. mBio. 2020. PMID: 32788381 Free PMC article.
-
Systematic literature review on the safety and immunogenicity of rotavirus vaccines when co-administered with meningococcal vaccines.Hum Vaccin Immunother. 2020 Nov 1;16(11):2861-2872. doi: 10.1080/21645515.2020.1739485. Epub 2020 Apr 16. Hum Vaccin Immunother. 2020. PMID: 32298219 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
