Multimodal General Anesthesia: Theory and Practice
- PMID: 30252709
- PMCID: PMC6203428
- DOI: 10.1213/ANE.0000000000003668
Multimodal General Anesthesia: Theory and Practice
Abstract
Balanced general anesthesia, the most common management strategy used in anesthesia care, entails the administration of different drugs together to create the anesthetic state. Anesthesiologists developed this approach to avoid sole reliance on ether for general anesthesia maintenance. Balanced general anesthesia uses less of each drug than if the drug were administered alone, thereby increasing the likelihood of its desired effects and reducing the likelihood of its side effects. To manage nociception intraoperatively and pain postoperatively, the current practice of balanced general anesthesia relies almost exclusively on opioids. While opioids are the most effective antinociceptive agents, they have undesirable side effects. Moreover, overreliance on opioids has contributed to the opioid epidemic in the United States. Spurred by concern of opioid overuse, balanced general anesthesia strategies are now using more agents to create the anesthetic state. Under these approaches, called "multimodal general anesthesia," the additional drugs may include agents with specific central nervous system targets such as dexmedetomidine and ones with less specific targets, such as magnesium. It is postulated that use of more agents at smaller doses further maximizes desired effects while minimizing side effects. Although this approach appears to maximize the benefit-to-side effect ratio, no rational strategy has been provided for choosing the drug combinations. Nociception induced by surgery is the primary reason for placing a patient in a state of general anesthesia. Hence, any rational strategy should focus on nociception control intraoperatively and pain control postoperatively. In this Special Article, we review the anatomy and physiology of the nociceptive and arousal circuits, and the mechanisms through which commonly used anesthetics and anesthetic adjuncts act in these systems. We propose a rational strategy for multimodal general anesthesia predicated on choosing a combination of agents that act at different targets in the nociceptive system to control nociception intraoperatively and pain postoperatively. Because these agents also decrease arousal, the doses of hypnotics and/or inhaled ethers needed to control unconsciousness are reduced. Effective use of this strategy requires simultaneous monitoring of antinociception and level of unconsciousness. We illustrate the application of this strategy by summarizing anesthetic management for 4 representative surgeries.
Conflict of interest statement
Conflicts of Interest: See Disclosures at the end of the article.
Figures
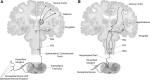
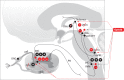
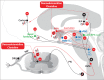
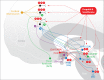
 denotes an excitatory effect. The symbol
denotes an excitatory effect. The symbol  denotes an inhibitory effect. The symbols
denotes an inhibitory effect. The symbols  and
and  denote inhibition of the indicated effects. ACh indicates acetylcholine; DRG, dorsal root ganglia; Glu, glutamate; LDT, laterodorsal tegmental area; mPRF, medial pontine reticular formation; NE, norepinephrine; PAF, peripheral afferent fiber; PAG, periaqueductal gray; PN, projection neuron; PPT, pedunculopontine tegmental area; RVM, rostral ventral medulla.
denote inhibition of the indicated effects. ACh indicates acetylcholine; DRG, dorsal root ganglia; Glu, glutamate; LDT, laterodorsal tegmental area; mPRF, medial pontine reticular formation; NE, norepinephrine; PAF, peripheral afferent fiber; PAG, periaqueductal gray; PN, projection neuron; PPT, pedunculopontine tegmental area; RVM, rostral ventral medulla.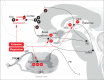
Comment in
-
Multimodal General Anesthesia: A Principled Approach to Producing the Drug-Induced, Reversible Coma of Anesthesia.Anesth Analg. 2018 Nov;127(5):1104-1106. doi: 10.1213/ANE.0000000000003743. Anesth Analg. 2018. PMID: 30335656 No abstract available.
-
Is the Balance in Anesthesia Right? Multitarget Approach and Alteration of Systemic Inflammation.Anesth Analg. 2019 Jun;128(6):e130. doi: 10.1213/ANE.0000000000004167. Anesth Analg. 2019. PMID: 31094827 No abstract available.
-
In Response.Anesth Analg. 2019 Jun;128(6):e131. doi: 10.1213/ANE.0000000000004168. Anesth Analg. 2019. PMID: 31094828 No abstract available.
References
-
- Lundy JS. Balanced anesthesia. Minn Med. 1926;9:399–404..
-
- Hendrickx JF, Eger EI, II, Sonner JM, Shafer SL. Is synergy the rule? A review of anesthetic interactions producing hypnosis and immobility. Anesth Analg. 2008;107:494–506.. - PubMed
-
- Purves D, Augustine GJ, Fitzpatrick D, Hall WC, Lamantia A, White LE. Purves D, Augustine GJ, Fitzpatrick D, Hall WC, Lamantia A, White LE. Pain. In: Neuroscience. 2012:5th ed Sunderland, MA: Sinauer Associates, Inc; 209–228..
-
- Lake APJ. Balanced anaesthesia 2005: avoiding the transition from acute to chronic pain. South Afr J Anaesth Analg. 2005;11:14–18..
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

