Time-resolved proteomics of adenovirus infected cells
- PMID: 30252905
- PMCID: PMC6155545
- DOI: 10.1371/journal.pone.0204522
Time-resolved proteomics of adenovirus infected cells
Abstract
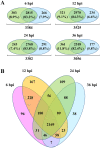
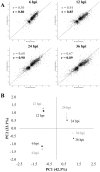
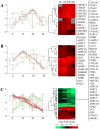
Viral infections cause large problems in the world and deeper understanding of the disease mechanisms is needed. Here we present an analytical strategy to investigate the host cell protein changes during human adenovirus type 2 (HAdV-C2 or Ad2) infection of lung fibroblasts by stable isotope labelling of amino acids in cell culture (SILAC) and nanoLC-MS/MS. This work focuses on early phase of infection (6 and 12 h post-infection (hpi)) but the data is combined with previously published late phase (24 and 36 hpi) proteomics data to produce a time series covering the complete infection. As many as 2169 proteins were quantitatively monitored from 6 to 36 hpi, while some proteins were time-specific. After applying different filter criteria, 2027 and 2150 proteins were quantified at 6 and 12 hpi and among them, 431 and 544 were significantly altered at the two time points. Pathway analysis showed that the De novo purine and pyrimidine biosynthesis, Glycolysis and Cytoskeletal regulation by Rho GTPase pathways were activated early during infection while inactivation of the Integrin signalling pathway started between 6 and 12 hpi. Moreover, upstream regulator analysis predicted MYC to be activated with time of infection and protein and RNA data for genes controlled by this transcription factor showed good correlation, which validated the use of protein data for this prediction. Among the identified phosphorylation sites, a group related to glycolysis and cytoskeletal reorganization were up-regulated during infection. The results show specific aspects on how the host cell proteins, the final products in the genetic information flow, are influenced by Ad2 infection, which would be overlooked if only knowledge derived from mRNA data is considered.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
References
-
- Bagchi S, Raychaudhuri P, Nevins JR. Adenovirus E1A proteins can dissociate heteromeric complexes involving the E2F transcription factor: a novel mechanism for E1A trans-activation. Cell. 1990;62: 659–669. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

