Colonic levels of vasoactive intestinal peptide decrease during infection and exogenous VIP protects epithelial mitochondria against the negative effects of IFNγ and TNFα induced during Citrobacter rodentium infection
- PMID: 30252907
- PMCID: PMC6155558
- DOI: 10.1371/journal.pone.0204567
Colonic levels of vasoactive intestinal peptide decrease during infection and exogenous VIP protects epithelial mitochondria against the negative effects of IFNγ and TNFα induced during Citrobacter rodentium infection
Abstract
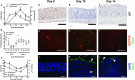
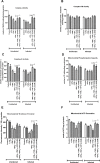
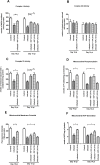
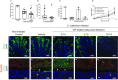
Citrobacter rodentium infection is a model for infection with attaching and effacing pathogens, such as enteropathogenic Escherichia coli. The vasoactive intestinal peptide (VIP) has emerged as an anti-inflammatory agent, documented to inhibit Th1 immune responses and successfully treat animal models of inflammation. VIP is also a mucus secretagogue. Here, we found that colonic levels of VIP decrease during murine C. rodentium infection with a similar time dependency as measurements reflecting mitochondrial function and epithelial integrity. The decrease in VIP appears mainly driven by changes in the cytokine environment, as no changes in VIP levels were detected in infected mice lacking interferon gamma (IFNγ). VIP supplementation alleviated the reduction of activity and levels of mitochondrial respiratory complexes I and IV, mitochondrial phosphorylation capacity, transmembrane potential and ATP generation caused by IFNγ, TNFα and C. rodentium infection, in an in vitro mucosal surface. Similarly, VIP treatment regimens that included the day 5-10 post infection period alleviated decreases in enzyme complexes I and IV, phosphorylation capacity, mitochondrial transmembrane potential and ATP generation as well as increased apoptosis levels during murine infection with C. rodentium. However, VIP treatment failed to alleviate colitis, although there was a tendency to decreased pathogen density in contact with the epithelium and in the spleen. Both in vivo and in vitro, NO generation increased during C. rodentium infection, which was alleviated by VIP. Thus, therapeutic VIP administration to restore the decreased levels during infection had beneficial effects on epithelial cells and their mitochondria, but not on the overall infection outcome.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures




References
-
- Barthold SW. The microbiology of transmissible murine colonic hyperplasia. Lab Anim Sci. 1980; 30: 167–173. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

