A synthetic glycosaminoglycan reduces sinonasal inflammation in a murine model of chronic rhinosinusitis
- PMID: 30252910
- PMCID: PMC6155557
- DOI: 10.1371/journal.pone.0204709
A synthetic glycosaminoglycan reduces sinonasal inflammation in a murine model of chronic rhinosinusitis
Abstract
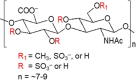
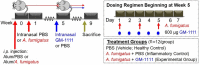
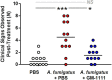
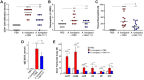
Chronic rhinosinusitis (CRS) is characterized by sustained mucosal inflammation, impaired mucociliary clearance, loss of cilia and epithelial barrier breakdown, and tissue remodeling. Certain glycosaminoglycans inhibit various inflammatory mediators, suppress bacterial growth, and provide important functions in mucosal tissue repair and mucociliary clearance. Herein, we evaluated the effects of a synthetic glycosaminoglycan, GM-1111, on the clinical signs and inflammatory tissue changes associated with CRS in mice. CRS was generated by repeated intranasal applications of Aspergillus fumigatus (A. fumigatus) extracts over 4 weeks. Mice were then intranasally administered GM-1111 (600 μg per dose, 5 times a week) or vehicle (phosphate buffered saline, PBS) for an additional 4 weeks while still being given A. fumigatus extracts to maintain a chronic inflammatory environment with acute exacerbations. Clinical signs indicative of sinonasal inflammation were recorded throughout the study. After 9 weeks, whole blood and sinonasal tissues were harvested for hematological, histological, and biochemical examination. The clinical signs, white blood cell counts, tissue markers of sinonasal inflammation, and histological changes caused by A. fumigatus extract administration were compared to the healthy (PBS vehicle) and GM-1111-treated groups (n = 12 per treatment group). Compared to vehicle-treated animals, animals treated with GM-1111 demonstrated significant reductions in clinical signs (p<0.05), degenerative tissue changes, goblet cell hyperplasia, inflammatory cell infiltration (p<0.01), innate immunity- (tlr2, tlr4, myd88, il1b, tnfa, il6, and il12) and adaptive immunity-associated (ccl11, ccl24, ccl5, il4, il5, and il13) cytokine gene expression (p<0.05 to p<0.0001) in sinonasal tissues, and serum IgE levels (p<0.01). Our data suggest that GM-1111 significantly reduces local and systemic effects of CRS-associated sinonasal inflammation.
Conflict of interest statement
WYL, JRS, and AP are employed by and GDP, TPK, and JAA have stock in GlycoMira Therapeutics, Inc. JAA is a paid consultant for Medtronic ENT, Spirox, and OptiNose. These affiliations do not alter the authors’ adherence to PLoS ONE policies on sharing data and materials.
Figures




Similar articles
-
Development and immunopathological characteristics of an Alternaria-induced chronic rhinosinusitis mouse model.PLoS One. 2020 Jun 16;15(6):e0234731. doi: 10.1371/journal.pone.0234731. eCollection 2020. PLoS One. 2020. PMID: 32544181 Free PMC article.
-
Prevention of sinonasal inflammation by a synthetic glycosaminoglycan.Int Forum Allergy Rhinol. 2017 Feb;7(2):177-184. doi: 10.1002/alr.21865. Epub 2016 Nov 11. Int Forum Allergy Rhinol. 2017. PMID: 27863138 Free PMC article.
-
Quercetin alleviates the progression of chronic rhinosinusitis by affecting nasal mucosal epithelial remodeling, inflammation, and Treg/Th17 imbalance.Biochem Cell Biol. 2025 Jan 1;103:1-11. doi: 10.1139/bcb-2025-0049. Biochem Cell Biol. 2025. PMID: 40602622
-
Mucosal Inflammatory Memory in Chronic Rhinosinusitis.Cells. 2024 Nov 23;13(23):1947. doi: 10.3390/cells13231947. Cells. 2024. PMID: 39682698 Free PMC article. Review.
-
Pathogenesis of chronic rhinosinusitis.Curr Allergy Asthma Rep. 2006 Nov;6(6):487-94. doi: 10.1007/s11882-006-0026-3. Curr Allergy Asthma Rep. 2006. PMID: 17049142 Review.
Cited by
-
Development and immunopathological characteristics of an Alternaria-induced chronic rhinosinusitis mouse model.PLoS One. 2020 Jun 16;15(6):e0234731. doi: 10.1371/journal.pone.0234731. eCollection 2020. PLoS One. 2020. PMID: 32544181 Free PMC article.
-
GM-1111 reduces radiation-induced oral mucositis in mice by targeting pattern recognition receptor-mediated inflammatory signaling.PLoS One. 2021 Mar 26;16(3):e0249343. doi: 10.1371/journal.pone.0249343. eCollection 2021. PLoS One. 2021. PMID: 33770116 Free PMC article.
-
Neutrophil Elastase and Chronic Lung Disease.Biomolecules. 2021 Jul 21;11(8):1065. doi: 10.3390/biom11081065. Biomolecules. 2021. PMID: 34439732 Free PMC article. Review.
-
Polysulfated Hyaluronan GlycoMira-1111 Inhibits Elastase and Improves Rheology in Cystic Fibrosis Sputum.Am J Respir Cell Mol Biol. 2021 Feb;64(2):260-267. doi: 10.1165/rcmb.2020-0157OC. Am J Respir Cell Mol Biol. 2021. PMID: 33264072 Free PMC article.
-
Host-microbe interactions in chronic rhinosinusitis biofilms and models for investigation.Biofilm. 2023 Sep 29;6:100160. doi: 10.1016/j.bioflm.2023.100160. eCollection 2023 Dec 15. Biofilm. 2023. PMID: 37928619 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

