Improved glycemic control with minimal systemic metformin exposure: Effects of Metformin Delayed-Release (Metformin DR) targeting the lower bowel over 16 weeks in a randomized trial in subjects with type 2 diabetes
- PMID: 30252913
- PMCID: PMC6155522
- DOI: 10.1371/journal.pone.0203946
Improved glycemic control with minimal systemic metformin exposure: Effects of Metformin Delayed-Release (Metformin DR) targeting the lower bowel over 16 weeks in a randomized trial in subjects with type 2 diabetes
Abstract
Objective: Metformin use is restricted in patients with renal impairment due to potential excess systemic accumulation. This study evaluated the glycemic effects and safety of metformin delayed-release (Metformin DR), which targets metformin delivery to the ileum to leverage its gut-based mechanisms of action while minimizing systemic exposure.
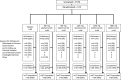
Research designs and methods: Participants (T2DM [HbA1c 7-10.5%], eGFR ≥60 mL/min/1.73m2, not taking metformin for ≥2 months) were randomized to QD placebo (PBO); QD Metformin DR 600, 900, 1200, or 1500 mg; or to single-blind BID Metformin immediate-release (IR) 1000 mg. The primary endpoint was change in HbA1c for Metformin DR vs. PBO at 16 weeks in the modified intent-to-treat (mITT) population (≥ 1 post-baseline HbA1c while on study drug), using a mixed-effects repeated measures model.
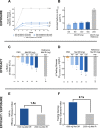
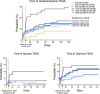
Results: 571 subjects were randomized (56 years, 53% male, 80% white; BMI 32.2±5.5 kg/m2; HbA1c 8.6±0.9%; 51% metformin naive); 542 were in the mITT population. Metformin DR 1200 and 1500 mg significantly reduced HbA1c (-0.49±0.13% and -0.62±0.12%, respectively, vs. PBO -0.06±0.13%; p<0.05) and FPG (Caverage Weeks 4-16: -22.3±4.2 mg/dL and -25.1±4.1 mg/dL, respectively vs. -2.5±4.2 mg/dL p<0.05). Metformin IR elicited greater HbA1c improvement (-1.10±0.13%; p<0.01 vs. Placebo and all doses of Metformin DR) but with ~3-fold greater plasma metformin exposure. Normalizing efficacy to systemic exposure, glycemic improvements with Metformin DR were 1.5-fold (HbA1c) and 2.1-fold (FPG) greater than Metformin IR. Adverse events were primarily gastrointestinal but these were less frequent with Metformin DR (<16% incidence) vs. Metformin IR (28%), particularly nausea (1-3% vs 10%).
Conclusion: Metformin DR exhibited greater efficacy per unit plasma exposure than Metformin IR. Future studies will evaluate the effects of Metformin DR in patients with type 2 diabetes and advanced renal disease.
Trial registration: Clinicaltrials.gov NCT02526524.
Conflict of interest statement
RRH is a consultant and/or advisor for Alere/Abbott, AstraZeneca, Boehringer Ingelheim/Lilly, Bristol Myers Squibb, Elcelyx, Ionis, Intarcia, Janssen, Lexicon, Ligand, Merck, Novo Nordisk, Sanofi, Sanofi Regeneron, and Servier; and has received research grants from Astareal, AstraZeneca, Lexicon, Lilly, ViaCyte, NIH-NIDDK, Novo Nordisk, VA NODES and Xeris. JPF has received research support from AbbVie, Allergan, AstraZeneca, Boehringer Ingelheim, BMS, Elcelyx, Eli Lilly, Genentech, IONIS, Janssen, Johnson and Johnson, Lexicon, Ligand, Madrigal, Merck, Mylan, Myovant, Novartis, Novo Nordisk, Ogeda, Pfizer, Sanofi, TaiwanJ, Theracos, and Viking; and has participated in advisory boards and consulting with AstraZeneca, BMS, Echosens, Elcelyx, Johnson and Johnson, Ligand, Novo Nordisk, and Sanofi. MF, JH, SS, CB, TB, AB, and BW are employees of and hold stock in Elcelyx Therapeutics. No other potential conflicts of interest relevant to this article were reported. The sponsor (Elcelyx Therapeutics) contributed to study design, data collection, analysis, writing the report, and the decision to submit the report for publication; this does not alter adherence to PLOS ONE policies on sharing data and materials.
Figures
References
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

