Moderate Heat Application Enhances the Efficacy of Nanosecond Pulse Stimulation for the Treatment of Squamous Cell Carcinoma
- PMID: 30253713
- PMCID: PMC6156209
- DOI: 10.1177/1533033818802305
Moderate Heat Application Enhances the Efficacy of Nanosecond Pulse Stimulation for the Treatment of Squamous Cell Carcinoma
Abstract
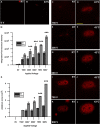
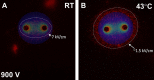
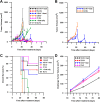
Nanosecond pulse stimulation as a tumor ablation therapy has been studied for the treatment of various carcinomas in animal models and has shown a significant survival benefit. In the current study, we found that moderate heating at 43°C for 2 minutes significantly enhanced in vitro nanosecond pulse stimulation-induced cell death of KLN205 murine squamous cell carcinoma cells by 2.43-fold at 600 V and by 2.32-fold at 900 V, as evidenced by propidium iodide uptake. Furthermore, the ablation zone in KLN205 cells placed in a 3-dimensional cell-culture model and pulsed at a voltage of 900 V at 43°C was 3 times larger than in cells exposed to nanosecond pulse stimulation at room temperature. Application of moderate heating alone did not cause cell death. A nanosecond pulse stimulation electrode with integrated controllable laser heating was developed to treat murine ectopic squamous cell carcinoma. With this innovative system, we were able to quickly heat and maintain the temperature of the target tumor at 43°C during nanosecond pulse stimulation. Nanosecond pulse stimulation with moderate heating was shown to significantly extend overall survival, delay tumor growth, and achieve a high rate of complete tumor regression. Moderate heating extended survival nearly 3-fold where median overall survival was 22 days for 9.8 kV without moderate heating and over 63 days for tumors pulsed with 600, 100 ns pulses at 5 Hz, at voltage of 9.8 kV with moderate heating. Median overall survival in the control groups was 24 and 31 days for mice with untreated tumors and tumors receiving moderate heat alone, respectively. Nearly 69% (11 of 16) of tumor-bearing mice treated with nanosecond pulse stimulation with moderate heating were tumor free at the completion of the study, whereas complete tumor regression was not observed in the control groups and in 9.8 kV without moderate heating. These results suggest moderate heating can reduce the necessary applied voltage for tumor ablation with nanosecond pulse stimulation.
Keywords: ablation; electroporation; irreversible electroporation; nanosecond pulse electric field; nanosecond pulse stimulation.
Conflict of interest statement
Figures

References
-
- Que SKT, Zwald FO, Schmults CD. Cutaneous squamous cell carcinoma: incidence, risk factors, diagnosis, and staging. J Am Acad Dermatol. 2018;78(2):237–247. - PubMed
-
- van Meerbeeck JP, Fennell DA, De Ruysscher DK. Small-cell lung cancer. Lancet. 2011;378(9804):1741–1755. - PubMed
-
- Scheffer HJ, Nielsen K, de Jong MC, et al. Irreversible electroporation for nonthermal tumor ablation in the clinical setting: a systematic review of safety and efficacy. J Vasc Interv Radiol. 2014;25(7):997–1011; quiz 1011. - PubMed
-
- Jiang C, Davalos RV, Bischof JC. A review of basic to clinical studies of irreversible electroporation therapy. IEEE Trans Biomed Eng. 2015;62(1):4–20. - PubMed
-
- Paiella S, Butturini G, Frigerio I, et al. Safety and feasibility of irreversible electroporation (IRE) in patients with locally advanced pancreatic cancer: results of a prospective study. Digest Surg. 2015;32(2):90–97. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

