Synaptic coupling of inner ear sensory cells is controlled by brevican-based extracellular matrix baskets resembling perineuronal nets
- PMID: 30253762
- PMCID: PMC6156866
- DOI: 10.1186/s12915-018-0566-8
Synaptic coupling of inner ear sensory cells is controlled by brevican-based extracellular matrix baskets resembling perineuronal nets
Abstract
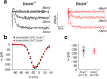
Background: Perineuronal nets (PNNs) are specialized aggregations of extracellular matrix (ECM) molecules surrounding specific neurons in the central nervous system (CNS). PNNs are supposed to control synaptic transmission and are frequently associated with neurons firing at high rates, including principal neurons of auditory brainstem nuclei. The origin of high-frequency activity of auditory brainstem neurons is the indefatigable sound-driven transmitter release of inner hair cells (IHCs) in the cochlea.
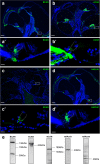
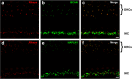
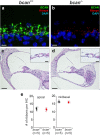
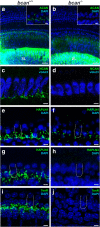
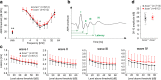
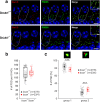
Results: Here, we show that synaptic poles of IHCs are ensheathed by basket-like ECM complexes formed by the same molecules that constitute PNNs of neurons in the CNS, including brevican, aggreccan, neurocan, hyaluronan, and proteoglycan link proteins 1 and 4 and tenascin-R. Genetic deletion of brevican, one of the main components, resulted in a massive degradation of ECM baskets at IHCs, a significant impairment in spatial coupling of pre- and postsynaptic elements and mild impairment of hearing.
Conclusions: These ECM baskets potentially contribute to control of synaptic transmission at IHCs and might be functionally related to PNNs of neurons in the CNS.
Keywords: Brevican; Cochlea; Extracellular matrix; Inner hair cell; Perineuronal net; Ribbon synapse.
Conflict of interest statement
Ethics approval
All experiments were carried out in accordance with the German law on the use of the laboratory animals and were approved by the Saxonian District Government, Leipzig (T27/16) and the regional board for scientific animal experiments of Saarland University.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures


Comment in
-
Brevican "nets" voltage-gated calcium channels at the hair cell ribbon synapse.BMC Biol. 2018 Sep 26;16(1):105. doi: 10.1186/s12915-018-0575-7. BMC Biol. 2018. PMID: 30253757 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

