Early hemodynamic performance of the Trifecta™ surgical bioprosthesis aortic valve in Indian patient population: 12 month outcomes of the EVEREST post-market study
- PMID: 30253784
- PMCID: PMC6157043
- DOI: 10.1186/s13019-018-0783-9
Early hemodynamic performance of the Trifecta™ surgical bioprosthesis aortic valve in Indian patient population: 12 month outcomes of the EVEREST post-market study
Abstract
Background: Indian patients undergoing surgical aortic valve replacement (SAVR) differ from western populations with respect to aortic annulus size and valve disease morphology. The purpose of this post-market, non-randomized observational study was to evaluate the early hemodynamic performance of the Trifecta™ bioprosthesis (Abbott, previously St. Jude Medical, Minneapolis, US) in an Indian patient population.
Methods: From January 2014 to September 2015, 100 patients (mean age 64.4 ± 7.1 years, 62% male) undergoing SAVR for valve disease (68% stenosis, 7% insufficiency, 25% mixed pathology) were enrolled across 10 centers in India. Patients implanted with a 19-27 mm Trifecta™ valve were eligible to participate and were prospectively followed for 12-months post-implantation. Echocardiographic hemodynamic performance was evaluated at pre-implant, pre-discharge and at 12-months by an independent core laboratory. Adverse events were adjudicated by the study sponsor. Functional status at 12-months was assessed according to NYHA classification. Continuous data was summarized using descriptive statistics (mean &standard deviation,) and categorical data was summarized using frequencies and percentages.
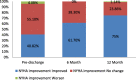
Result: Ninety patients (mean age 64.5, 62.2% male) completed the 12-month follow up. Significant improvements in hemodynamic valve performance were reported in 81 patients with available echocardiographic data at 12 months. Compared to baseline at 12-month follow up visit, mean effective orifice area increased from 0.75cm2 to 1.61cm2 (p < 0.0001), mean pressure gradient reduced to 10.42 mmHg from 51.47 mmHg (p < 0.0001), cardiac output increased from 4.46 l/min to 4.85 l/min (P 0.9254). Compared to baseline, functional status improved by ≥1 NYHA class in 75% of patients at 12 months (95% Clopper-Pearson (Exact) confidence limit [64.6%, 83.6%]). No instances of early mortality (< 30 days from index procedure) or structural valve dysfunction were reported.
Conclusion: In an Indian patient population, implantation of the Trifecta™ bioprosthesis is shown to be safe and associated with favorable early hemodynamic performance and improved functional status at 12 months.
Trial registration: The clinical study has been registered under Clinical Trial Registry-India ( http://www.ctri.nic.in ) and registration number is CTRI/2014/02/004434 registered on 25 February 2014 retrospectively registered.
Keywords: Aortic valve replacement; Stented bioprosthesis; Structural valve dysfunction; Valvular disease.
Conflict of interest statement
Ethics approval and consent to participate
The study was conducted in accordance with the Declaration of Helsinki principals and approved by the Institutional Ethics Committee of Madras Medical Mission, Institutional Ethics Committee of Care Institute of Medical Sciences, Institutional Ethics Committee of Star Hospital, Institutional Ethics Committee of Apollo Gleneagles Hospital, Institutional Ethics Committee of Fortis Hospital, Institutional Ethics Committee of PD Hinduja Hospital and Research Center, Sal Ethics Committee of Sal Hospital, The Independent Ethics Committee of Fortis Escorts Heart Institute, The Institutional Ethics Committee of Max Super Speciality Hospital and Yashoda Academy of Medical Education and Research Institutional Ethics Committee of Yashoda Hospital. Informed consent (Consent to Participate) was obtained from each of the patients.
Consent for publication
While explaining the study and obtaining consent for participation, participants were told that the results of this research will be published and their identities will be kept confidential.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures
References
-
- Talwar S, Sharma AK, Kumar AS. Tissue heart valve implantation in India; Indications, results and impact on quality of life. IJTCVS. Tissue valves. 2008;24:10–14.
-
- Joshi K, et al. Aortic valve replacement in predominant aortic stenosis: what is an appropriate size valve? IJTCVS. 2007;23:141–145.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources