Polyamine function in archaea and bacteria
- PMID: 30254075
- PMCID: PMC6290158
- DOI: 10.1074/jbc.TM118.005670
Polyamine function in archaea and bacteria
Abstract
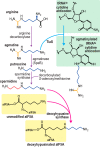
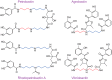
Most of the phylogenetic diversity of life is found in bacteria and archaea, and is reflected in the diverse metabolism and functions of bacterial and archaeal polyamines. The polyamine spermidine was probably present in the last universal common ancestor, and polyamines are known to be necessary for critical physiological functions in bacteria, such as growth, biofilm formation, and other surface behaviors, and production of natural products, such as siderophores. There is also phylogenetic diversity of function, indicated by the role of polyamines in planktonic growth of different species, ranging from absolutely essential to entirely dispensable. However, the cellular molecular mechanisms responsible for polyamine function in bacterial growth are almost entirely unknown. In contrast, the molecular mechanisms of essential polyamine functions in archaea are better understood: covalent modification by polyamines of translation factor aIF5A and the agmatine modification of tRNAIle As with bacterial hyperthermophiles, archaeal thermophiles require long-chain and branched polyamines for growth at high temperatures. For bacterial species in which polyamines are essential for growth, it is still unknown whether the molecular mechanisms underpinning polyamine function involve covalent or noncovalent interactions. Understanding the cellular molecular mechanisms of polyamine function in bacterial growth and physiology remains one of the great challenges for future polyamine research.
Keywords: agmatine; archaea; bacterial metabolism; biofilm; biosynthesis; branched-chain polyamine; cell growth; deoxyhypusine; halophile; homospermidine; phylogenetics; polyamine; post-translational modification (PTM); putrescine; siderophore; spermidine; thermophile; translation.
© 2018 Michael.
Conflict of interest statement
The author declares that he has no conflicts of interest with the contents of this article
Figures



References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

