ATG16L1 orchestrates interleukin-22 signaling in the intestinal epithelium via cGAS-STING
- PMID: 30254094
- PMCID: PMC6219748
- DOI: 10.1084/jem.20171029
ATG16L1 orchestrates interleukin-22 signaling in the intestinal epithelium via cGAS-STING
Abstract
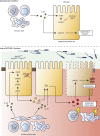
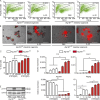
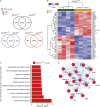
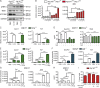
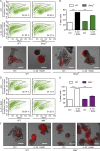
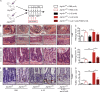
A coding variant of the inflammatory bowel disease (IBD) risk gene ATG16L1 has been associated with defective autophagy and deregulation of endoplasmic reticulum (ER) function. IL-22 is a barrier protective cytokine by inducing regeneration and antimicrobial responses in the intestinal mucosa. We show that ATG16L1 critically orchestrates IL-22 signaling in the intestinal epithelium. IL-22 stimulation physiologically leads to transient ER stress and subsequent activation of STING-dependent type I interferon (IFN-I) signaling, which is augmented in Atg16l1 ΔIEC intestinal organoids. IFN-I signals amplify epithelial TNF production downstream of IL-22 and contribute to necroptotic cell death. In vivo, IL-22 treatment in Atg16l1 ΔIEC and Atg16l1 ΔIEC/Xbp1 ΔIEC mice potentiates endogenous ileal inflammation and causes widespread necroptotic epithelial cell death. Therapeutic blockade of IFN-I signaling ameliorates IL-22-induced ileal inflammation in Atg16l1 ΔIEC mice. Our data demonstrate an unexpected role of ATG16L1 in coordinating the outcome of IL-22 signaling in the intestinal epithelium.
© 2018 Aden et al.
Figures




References
-
- Aden K., Rehman A., Falk-Paulsen M., Secher T., Kuiper J., Tran F., Pfeuffer S., Sheibani-Tezerji R., Breuer A., Luzius A., et al. 2016. Epithelial IL-23R Signaling Licenses Protective IL-22 Responses in Intestinal Inflammation. Cell Reports. 16:2208–2218. 10.1016/j.celrep.2016.07.054 - DOI - PMC - PubMed
-
- Bachmann M., Horn K., Rudloff I., Goren I., Holdener M., Christen U., Darsow N., Hunfeld K.-P., Koehl U., Kind P., et al. 2010. Early production of IL-22 but not IL-17 by peripheral blood mononuclear cells exposed to live Borrelia burgdorferi: the role of monocytes and interleukin-1. PLoS Pathog. 6:e1001144 10.1371/journal.ppat.1001144 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

