A pilot study of lower doses of ibrutinib in patients with chronic lymphocytic leukemia
- PMID: 30254130
- PMCID: PMC6251009
- DOI: 10.1182/blood-2018-06-860593
A pilot study of lower doses of ibrutinib in patients with chronic lymphocytic leukemia
Abstract
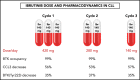
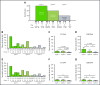
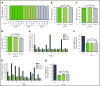
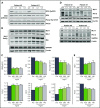
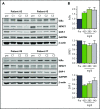
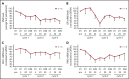
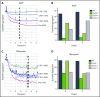
Ibrutinib is highly efficacious and used at 420 mg/d for treatment of chronic lymphocytic leukemia (CLL). We previously demonstrated a decline in Bruton's tyrosine kinase (BTK) protein levels in CLL cells after 1 cycle of ibrutinib, suggesting ibrutinib dose could be lowered after the first cycle without loss of biological effect. To test this postulate, a pilot study (NCT02801578) was designed to systematically reduce ibrutinib dosing within the same patient with CLL over the course of three 28-day cycles. After an initial cycle of 420 mg/d, the dose was reduced to 280 mg/d in cycle 2, and then to 140 mg/d in cycle 3. Eleven patients began study treatment, and 9 completed the 3 cycles. Plasma and intracellular pharmacokinetics (PK), BTK occupancy, and pharmacodynamic (PD) response at different doses of ibrutinib were compared. Plasma and intracellular levels of ibrutinib were dose-dependent, and even the lowest dose was sufficient to occupy, on average, more than 95% of BTK protein. In concert, BTK downstream signaling inhibition was maintained with 140 mg/d ibrutinib in cycle 3, and there were comparable reductions in total and phospho-BTK (Tyr223) protein levels across 3 cycles. Reductions of plasma chemokine CCL3 and CCL4 levels, considered to be biomarkers of ibrutinib response, were similar during the 3 cycles. These PK/PD data demonstrate that after 1 cycle of ibrutinib at the standard 420 mg/d dose, the dose can be reduced without losing biological activity. Clinical efficacy of lower doses needs to be systematically evaluated. Such dose reductions would lower drug cost, lessen untoward toxicity, and facilitate rationale-based combinations. This trial was registered at www.clinicaltrials.gov as #NCT02801578.
© 2018 by The American Society of Hematology.
Conflict of interest statement
Conflict-of-interest disclosure: V.G., N.J., P.A.T., and W.G.W. have received research funding from Pharmacyclics for previous investigations. The remaining authors declare no competing financial interests.
Figures
Comment in
-
Is less equal with ibrutinib dose?Blood. 2018 Nov 22;132(21):2211-2212. doi: 10.1182/blood-2018-10-878579. Blood. 2018. PMID: 30467185 No abstract available.
-
Ibrutinib dose intensity in high-risk chronic lymphocytic leukemia.Hematol Oncol. 2022 Dec;40(5):1100-1104. doi: 10.1002/hon.3032. Epub 2022 Jun 1. Hematol Oncol. 2022. PMID: 35613703 No abstract available.
References
-
- Imbruvica - Prescribing information. https://www.accessdata.fda.gov/drugsatfda_docs/label/2015/205552s002lbl.pdf. Accessed 1 June 2018.
-
- McMullen JR, Boey EJ, Ooi JY, Seymour JF, Keating MJ, Tam CS. Ibrutinib increases the risk of atrial fibrillation, potentially through inhibition of cardiac PI3K-Akt signaling. Blood. 2014;124(25):3829-3830. - PubMed
-
- Kamel S, Horton L, Ysebaert L, et al. Ibrutinib inhibits collagen-mediated but not ADP-mediated platelet aggregation. Leukemia. 2015;29(4):783-787. - PubMed
-
- Levade M, David E, Garcia C, et al. Ibrutinib treatment affects collagen and von Willebrand factor-dependent platelet functions. Blood. 2014;124(26):3991-3995. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

