Follow-on RifAximin for the Prevention of recurrence following standard treatment of Infection with Clostridium Difficile (RAPID): a randomised placebo controlled trial
- PMID: 30254135
- PMCID: PMC6582824
- DOI: 10.1136/gutjnl-2018-316794
Follow-on RifAximin for the Prevention of recurrence following standard treatment of Infection with Clostridium Difficile (RAPID): a randomised placebo controlled trial
Abstract
Background: Clostridium difficile infection (CDI) recurs after initial treatment in approximately one in four patients. A single-centre pilot study suggested that this could be reduced using 'follow-on' rifaximin treatment. We aimed to assess the efficacy of rifaximin treatment in preventing recurrence.
Methods: A multisite, parallel group, randomised, placebo controlled trial recruiting patients aged ≥18 years immediately after resolution of CDI through treatment with metronidazole or vancomycin. Participants received either rifaximin 400 mg three times a day for 2 weeks, reduced to 200 mg three times a day for a further 2 weeks or identical placebo. The primary endpoint was recurrence of CDI within 12 weeks of trial entry.
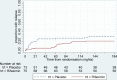
Results: Between December 2012 and March 2016, 151 participants were randomised to either rifaximin or placebo. Primary outcome data were available on 130. Mean age was 71.9 years (SD 15.3). Recurrence within 12 weeks was 29.5% (18/61) among participants allocated to placebo compared with 15.9% (11/69) among those allocated to rifaximin, a difference between groups of 13.7% (95% CI -28.1% to 0.7%, p=0.06). The risk ratio was 0.54 (95% CI 0.28 to 1.05, p=0.07). During 6-month safety follow-up, nine participants died in each group (12%). Adverse event rates were similar between groups.
Conclusion: While 'follow-on' rifaximin after CDI appeared to halve recurrence rate, we failed to reach our recruitment target in this group of frail elderly patients, so the estimated effect of rifaximin lacks precision. A meta-analysis including a previous trial suggests that rifaximin may be effective; however, further, larger confirmatory studies are needed.
Keywords: diarrhoea; enteric infections; infective colitis; inflammation.
© Author(s) (or their employer(s)) 2019. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: GM, LB, KS, MD, NB, AM, AJ, AS, NM declare no competing interests. RS received the IMP and matching placebo from Norgine Pharmaceuticals Ltd free of cost.
Figures

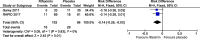
Comment in
-
Follow-on rifaximin for the prevention of recurrence following standard treatment of infection with Clostridium difficile: a competing risks analysis provides a full picture of possible treatment effects.Gut. 2020 Feb;69(2):398-400. doi: 10.1136/gutjnl-2018-317839. Epub 2018 Dec 28. Gut. 2020. PMID: 30593454 No abstract available.
References
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
