Pharmacologic Ascorbate Reduces Radiation-Induced Normal Tissue Toxicity and Enhances Tumor Radiosensitization in Pancreatic Cancer
- PMID: 30254147
- PMCID: PMC6295907
- DOI: 10.1158/0008-5472.CAN-18-1680
Pharmacologic Ascorbate Reduces Radiation-Induced Normal Tissue Toxicity and Enhances Tumor Radiosensitization in Pancreatic Cancer
Abstract
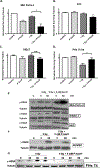
: Chemoradiation therapy is the mainstay for treatment of locally advanced, borderline resectable pancreatic cancer. Pharmacologic ascorbate (P-AscH-, i.e., intravenous infusions of ascorbic acid, vitamin C), but not oral ascorbate, produces high plasma concentrations capable of selective cytotoxicity to tumor cells. In doses achievable in humans, P-AscH- decreases the viability and proliferative capacity of pancreatic cancer via a hydrogen peroxide (H2O2)-mediated mechanism. In this study, we demonstrate that P-AscH- radiosensitizes pancreatic cancer cells but inhibits radiation-induced damage to normal cells. Specifically, radiation-induced decreases in clonogenic survival and double-stranded DNA breaks in tumor cells, but not in normal cells, were enhanced by P-AscH-, while radiation-induced intestinal damage, collagen deposition, and oxidative stress were also reduced with P-AscH- in normal tissue. We also report on our first-in-human phase I trial that infused P-AscH- during the radiotherapy "beam on." Specifically, treatment with P-AscH- increased median overall survival compared with our institutional average (21.7 vs. 12.7 months, P = 0.08) and the E4201 trial (21.7 vs. 11.1 months). Progression-free survival in P-AscH--treated subjects was also greater than our institutional average (13.7 vs. 4.6 months, P < 0.05) and the E4201 trial (6.0 months). Results indicated that P-AscH- in combination with gemcitabine and radiotherapy for locally advanced pancreatic adenocarcinoma is safe and well tolerated with suggestions of efficacy. Because of the potential effect size and minimal toxicity, our findings suggest that investigation of P-AscH- efficacy is warranted in a phase II clinical trial. SIGNIFICANCE: These findings demonstrate that pharmacologic ascorbate enhances pancreatic tumor cell radiation cytotoxicity in addition to offering potential protection from radiation damage in normal surrounding tissue, making it an optimal agent for improving treatment of locally advanced pancreatic adenocarcinoma.
©2018 American Association for Cancer Research.
Conflict of interest statement
Conflict of interest statement: The authors have no conflicts of interest to disclose.
Figures




References
-
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA: A Cancer Journal for Clinicians 2018;68(1):7–30. - PubMed
-
- Iacobuzio-Donahue CA, Fu B, Yachida S, Luo M, Abe H, Henderson CM, et al. DPC4 gene status of the primary carcinoma correlates with patterns of failure in patients with pancreatic cancer. Journal of clinical oncology : official journal of the American Society of Clinical Oncology 2009;27(11):1806–13. - PMC - PubMed
-
- Balaban EP, Mangu PB, Khorana AA, Shah MA, Mukherjee S, Crane CH, et al. Locally Advanced, Unresectable Pancreatic Cancer: American Society of Clinical Oncology Clinical Practice Guideline. Journal of Clinical Oncology 2016;34(22):2654–68. - PubMed
-
- Loehrer PJ Sr., Feng Y, Cardenes H, Wagner L, Brell JM, Cella D, et al. Gemcitabine alone versus gemcitabine plus radiotherapy in patients with locally advanced pancreatic cancer: an Eastern Cooperative Oncology Group trial. Journal of clinical oncology : official journal of the American Society of Clinical Oncology 2011;29(31):4105–12. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

