BRCA1-IRIS promotes human tumor progression through PTEN blockade and HIF-1α activation
- PMID: 30254159
- PMCID: PMC6187201
- DOI: 10.1073/pnas.1807112115
BRCA1-IRIS promotes human tumor progression through PTEN blockade and HIF-1α activation
Abstract
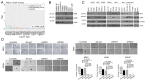
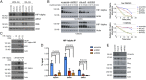
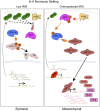
BRCA1 is an established breast and ovarian tumor suppressor gene that encodes multiple protein products whose individual contributions to human cancer suppression are poorly understood. BRCA1-IRIS (also known as "IRIS"), an alternatively spliced BRCA1 product and a chromatin-bound replication and transcription regulator, is overexpressed in various primary human cancers, including breast cancer, lung cancer, acute myeloid leukemia, and certain other carcinomas. Its naturally occurring overexpression can promote the metastasis of patient-derived xenograft (PDX) cells and other human cancer cells in mouse models. The IRIS-driven metastatic mechanism results from IRIS-dependent suppression of phosphatase and tensin homolog (PTEN) transcription, which in turn perturbs the PI3K/AKT/GSK-3β pathway leading to prolyl hydroxylase-independent HIF-1α stabilization and activation in a normoxic environment. Thus, despite the tumor-suppressing genetic origin of IRIS, its properties more closely resemble those of an oncoprotein that, when spontaneously overexpressed, can, paradoxically, drive human tumor progression.
Keywords: BRCA1-IRIS; HIF-1α; PTEN; metastasis.
Copyright © 2018 the Author(s). Published by PNAS.
Conflict of interest statement
Conflict of interest statement: D.M.L. is a grantee of and consultant to the Novartis Institute of Biomedical Research. W.G.K. owns equity in and consults for Lilly. A.L.K. was, during the course of some of this work, a grantee of and consultant to the Novartis Institute of Biomedical Research.
Figures




References
-
- Miki Y, et al. A strong candidate for the breast and ovarian cancer susceptibility gene BRCA1. Science. 1994;266:66–71. - PubMed
-
- ElShamy WM, Livingston DM. Identification of BRCA1-IRIS, a BRCA1 locus product. Nat Cell Biol. 2004;6:954–967. - PubMed
-
- Nakuci E, Mahner S, Direnzo J, ElShamy WM. BRCA1-IRIS regulates cyclin D1 expression in breast cancer cells. Exp Cell Res. 2006;312:3120–3131. - PubMed
-
- Paul BT, Blanchard Z, Ridgway M, ElShamy WM. BRCA1-IRIS inactivation sensitizes ovarian tumors to cisplatin. Oncogene. 2015;34:3036–3052. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

