Single-molecule analysis of endogenous β-actin mRNA trafficking reveals a mechanism for compartmentalized mRNA localization in axons
- PMID: 30254174
- PMCID: PMC6187124
- DOI: 10.1073/pnas.1806189115
Single-molecule analysis of endogenous β-actin mRNA trafficking reveals a mechanism for compartmentalized mRNA localization in axons
Abstract
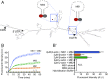
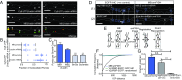
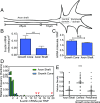
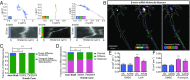
During embryonic nervous system assembly, mRNA localization is precisely regulated in growing axons, affording subcellular autonomy by allowing controlled protein expression in space and time. Different sets of mRNAs exhibit different localization patterns across the axon. However, little is known about how mRNAs move in axons or how these patterns are generated. Here, we couple molecular beacon technology with highly inclined and laminated optical sheet microscopy to image single molecules of identified endogenous mRNA in growing axons. By combining quantitative single-molecule imaging with biophysical motion models, we show that β-actin mRNA travels mainly as single copies and exhibits different motion-type frequencies in different axonal subcompartments. We find that β-actin mRNA density is fourfold enriched in the growth cone central domain compared with the axon shaft and that a modicum of directed transport is vital for delivery of mRNA to the axon tip. Through mathematical modeling we further demonstrate that directional differences in motor-driven mRNA transport speeds are sufficient to generate β-actin mRNA enrichment at the growth cone. Our results provide insight into how mRNAs are trafficked in axons and a mechanism for generating different mRNA densities across axonal subcompartments.
Keywords: RNA localization; axon; molecular beacon; single molecule; β-actin.
Copyright © 2018 the Author(s). Published by PNAS.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
The heterogeneous nuclear ribonucleoprotein-R is necessary for axonal beta-actin mRNA translocation in spinal motor neurons.Hum Mol Genet. 2010 May 15;19(10):1951-66. doi: 10.1093/hmg/ddq073. Epub 2010 Feb 18. Hum Mol Genet. 2010. PMID: 20167579
-
XMAP215 promotes microtubule-F-actin interactions to regulate growth cone microtubules during axon guidance in Xenopuslaevis.J Cell Sci. 2019 Apr 30;132(9):jcs224311. doi: 10.1242/jcs.224311. J Cell Sci. 2019. PMID: 30890650 Free PMC article.
-
Phosphorylation of zipcode binding protein 1 is required for brain-derived neurotrophic factor signaling of local beta-actin synthesis and growth cone turning.J Neurosci. 2010 Jul 14;30(28):9349-58. doi: 10.1523/JNEUROSCI.0499-10.2010. J Neurosci. 2010. PMID: 20631164 Free PMC article.
-
Xenopus laevis as a model system to study cytoskeletal dynamics during axon pathfinding.Genesis. 2017 Jan;55(1-2):10.1002/dvg.22994. doi: 10.1002/dvg.22994. Genesis. 2017. PMID: 28095612 Free PMC article. Review.
-
Regulation of actin filament assembly and disassembly in growth cone motility and axon guidance.Brain Res Bull. 2023 Jan;192:21-35. doi: 10.1016/j.brainresbull.2022.10.019. Epub 2022 Nov 3. Brain Res Bull. 2023. PMID: 36336143 Review.
Cited by
-
Microtubule-based transport is essential to distribute RNA and nascent protein in skeletal muscle.Nat Commun. 2021 Oct 27;12(1):6079. doi: 10.1038/s41467-021-26383-9. Nat Commun. 2021. PMID: 34707124 Free PMC article.
-
Decoding the complex journeys of RNAs along neurons.Nucleic Acids Res. 2025 Apr 10;53(7):gkaf293. doi: 10.1093/nar/gkaf293. Nucleic Acids Res. 2025. PMID: 40243060 Free PMC article.
-
Actin filaments form a size-dependent diffusion barrier around centrosomes.EMBO Rep. 2023 Jan 9;24(1):e54935. doi: 10.15252/embr.202254935. Epub 2022 Oct 31. EMBO Rep. 2023. PMID: 36314725 Free PMC article.
-
Axonal precursor miRNAs hitchhike on endosomes and locally regulate the development of neural circuits.EMBO J. 2020 Mar 16;39(6):e102513. doi: 10.15252/embj.2019102513. Epub 2020 Feb 19. EMBO J. 2020. PMID: 32073171 Free PMC article.
-
Cue-Polarized Transport of β-actin mRNA Depends on 3'UTR and Microtubules in Live Growth Cones.Front Cell Neurosci. 2018 Sep 10;12:300. doi: 10.3389/fncel.2018.00300. eCollection 2018. Front Cell Neurosci. 2018. PMID: 30250426 Free PMC article.
References
-
- Yao J, Sasaki Y, Wen Z, Bassell GJ, Zheng JQ. An essential role for beta-actin mRNA localization and translation in Ca2+-dependent growth cone guidance. Nat Neurosci. 2006;9:1265–1273. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

