Arginase 1 promotes retinal neurovascular protection from ischemia through suppression of macrophage inflammatory responses
- PMID: 30254218
- PMCID: PMC6156564
- DOI: 10.1038/s41419-018-1051-6
Arginase 1 promotes retinal neurovascular protection from ischemia through suppression of macrophage inflammatory responses
Abstract
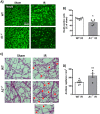
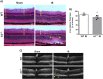
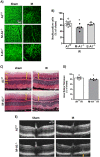
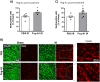
The lack of effective therapies to limit neurovascular injury in ischemic retinopathy is a major clinical problem. This study aimed to examine the role of ureohydrolase enzyme, arginase 1 (A1), in retinal ischemia-reperfusion (IR) injury. A1 competes with nitric oxide synthase (NOS) for their common substrate L-arginine. A1-mediated L-arginine depletion reduces nitric oxide (NO) formation by NOS leading to vascular dysfunction when endothelial NOS is involved but prevents inflammatory injury when inducible NOS is involved. Studies were performed using wild-type (WT) mice, global A1+/- knockout (KO), endothelial-specific A1 KO, and myeloid-specific A1 KO mice subjected to retinal IR injury. Global as well as myeloid-specific A1 KO mice showed worsened IR-induced neuronal loss and retinal thinning. Deletion of A1 in endothelial cells had no effect, while treatment with PEGylated (PEG) A1 improved neuronal survival in WT mice. In addition, A1+/- KO mice showed worsened vascular injury manifested by increased acellular capillaries. Western blotting analysis of retinal tissue showed increased inflammatory and necroptotic markers with A1 deletion. In vitro experiments showed that macrophages lacking A1 exhibit increased inflammatory response upon LPS stimulation. PEG-A1 treatment dampened this inflammatory response and decreased the LPS-induced metabolic reprogramming. Moreover, intravitreal injection of A1 KO macrophages or systemic macrophage depletion with clodronate liposomes increased neuronal loss after IR injury. These results demonstrate that A1 reduces IR injury-induced retinal neurovascular degeneration via dampening macrophage inflammatory responses. Increasing A1 offers a novel strategy for limiting neurovascular injury and promoting macrophage-mediated repair.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

