Microglial dopamine receptor elimination defines sex-specific nucleus accumbens development and social behavior in adolescent rats
- PMID: 30254300
- PMCID: PMC6156594
- DOI: 10.1038/s41467-018-06118-z
Microglial dopamine receptor elimination defines sex-specific nucleus accumbens development and social behavior in adolescent rats
Abstract
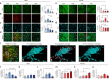
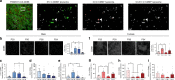
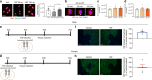
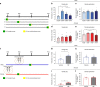
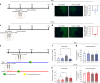
Adolescence is a developmental period in which the mesolimbic dopaminergic "reward" circuitry of the brain, including the nucleus accumbens (NAc), undergoes significant plasticity. Dopamine D1 receptors (D1rs) in the NAc are critical for social behavior, but how these receptors are regulated during adolescence is not well understood. In this report, we demonstrate that microglia and complement-mediated phagocytic activity shapes NAc development by eliminating D1rs in male, but not female rats, during adolescence. Moreover, immune-mediated elimination of D1rs is required for natural developmental changes in male social play behavior. These data demonstrate for the first time that microglia and complement-mediated immune signaling (i) participate in adolescent brain development in a sex-specific manner, and (ii) are causally implicated in developmental changes in behavior. These data have broad implications for understanding the adolescent critical period of development, the molecular mechanisms underlying social behavior, and sex differences in brain structure and function.
Conflict of interest statement
The authors declare no competing interests.
Figures
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

