TC2N, a novel oncogene, accelerates tumor progression by suppressing p53 signaling pathway in lung cancer
- PMID: 30254375
- PMCID: PMC6748156
- DOI: 10.1038/s41418-018-0202-8
TC2N, a novel oncogene, accelerates tumor progression by suppressing p53 signaling pathway in lung cancer
Abstract
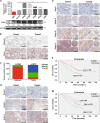
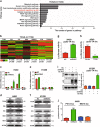
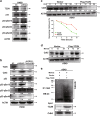
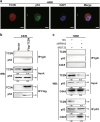
The protein containing the C2 domain has been well documented for its essential roles in endocytosis, cellular metabolism and cancer. Tac2-N (TC2N) is a tandem C2 domain-containing protein, but its function, including its role in tumorigenesis, remains unknown. Here, we first identified TC2N as a novel oncogene in lung cancer. TC2N was preferentially upregulated in lung cancer tissues compared with adjacent normal lung tissues. High TC2N expression was significantly associated with poor outcome of lung cancer patients. Knockdown of TC2N markedly induces cell apoptosis and cell cycle arrest with repressing proliferation in vitro, and suppresses tumorigenicity in vivo, whereas overexpression of TC2N has the opposite effects both in vitro and in vivo. Using a combination of TCGA database and bioinformatics, we demonstrate that TC2N is involved in regulation of the p53 signaling pathway. Mechanistically, TC2N attenuates p53 signaling pathway through inhibiting Cdk5-induced phosphorylation of p53 via inducing Cdk5 degradation or disrupting the interaction between Cdk5 and p53. Moreover, the blockade of p53 attenuates the function of TC2N knockdown in the regulation of cell proliferation and apoptosis. In addition, downregulated TC2N is involved in the apoptosis of lung cancer cells induced by doxorubicin, leading to p53 pathway activation. Overall, these findings uncover a role for the p53 inactivator TC2N in regulating the proliferation and apoptosis of lung cancer cells. Our present study provides novel insights into the mechanism of tumorigenesis in lung cancer.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures




References
-
- Zhang J, Gold KA, Lin HY, Swisher SG, Xing Y, Lee JJ, et al. Relationship between tumor size and survival in non-small-cell lung cancer (NSCLC): an analysis of the surveillance, epidemiology, and end results (SEER) registry. J Thorac Oncol. 2015;10:682–90. doi: 10.1097/JTO.0000000000000456. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

