Caspase-1 inhibition alleviates cognitive impairment and neuropathology in an Alzheimer's disease mouse model
- PMID: 30254377
- PMCID: PMC6156230
- DOI: 10.1038/s41467-018-06449-x
Caspase-1 inhibition alleviates cognitive impairment and neuropathology in an Alzheimer's disease mouse model
Abstract
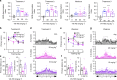
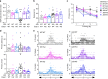
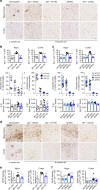
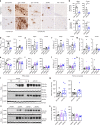
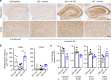
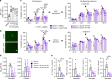
Alzheimer's disease (AD) is an intractable progressive neurodegenerative disease characterized by cognitive decline and dementia. An inflammatory neurodegenerative pathway, involving Caspase-1 activation, is associated with human age-dependent cognitive impairment and several classical AD brain pathologies. Here, we show that the nontoxic and blood-brain barrier permeable small molecule Caspase-1 inhibitor VX-765 dose-dependently reverses episodic and spatial memory impairment, and hyperactivity in the J20 mouse model of AD. Cessation of VX-765 results in the reappearance of memory deficits in the mice after 1 month and recommencement of treatment re-establishes normal cognition. VX-765 prevents progressive amyloid beta peptide deposition, reverses brain inflammation, and normalizes synaptophysin protein levels in mouse hippocampus. Consistent with these findings, Caspase-1 null J20 mice are protected from episodic and spatial memory deficits, neuroinflammation and Aβ accumulation. These results provide in vivo proof of concept for Caspase-1 inhibition against AD cognitive deficits and pathologies.
Conflict of interest statement
The authors declare no competing interests.
Figures

References
-
- Shi Qiaoqiao, Chowdhury Saba, Ma Rong, Le Kevin X., Hong Soyon, Caldarone Barbara J., Stevens Beth, Lemere Cynthia A. Complement C3 deficiency protects against neurodegeneration in aged plaque-rich APP/PS1 mice. Science Translational Medicine. 2017;9(392):eaaf6295. doi: 10.1126/scitranslmed.aaf6295. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

