A novel microfluidic liposomal formulation for the delivery of the SN-38 camptothecin: characterization and in vitro assessment of its cytotoxic effect on two tumor cell lines
- PMID: 30254436
- PMCID: PMC6141119
- DOI: 10.2147/IJN.S166219
A novel microfluidic liposomal formulation for the delivery of the SN-38 camptothecin: characterization and in vitro assessment of its cytotoxic effect on two tumor cell lines
Abstract
Purpose: Irinotecan (CPT-11) and SN-38 - its active metabolite - are alkaloid-derived topoisomerase I interactive compounds widely used in various cancer therapy protocols. To solve the problems associated with the instability of their lactone ring at physiological pH and with the extreme insolubility of SN-38, the development of delivery carriers (eg, liposomes) has been considered a subject of unquestionable medical interest. This article focuses on the development of an alternative protocol to the classical lipid-film hydration procedures to obtain a pharmaceutical formulation for SN-38.
Methods: SN-38-loaded liposomes (SN-38lip) were produced by microemulsification, without a prior lipid-film preparation step, and characterized by different methods. Formulation parameters were determined by photon correlation spectroscopy, and the SN-38 entrapment efficiency was evaluated by absorbance spectroscopy. SN-38lip was obtained as a dry, white powder by lyophilization. MTT and LDH assays were conducted to assess the cytotoxic effect of SN-38, both in liposomal (SN-38lip) and solubilized form (SN-38sol); flow cytometry was used to quantify SN-38 uptake and to analyze cell-cycle phase distribution after drug exposure.
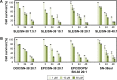
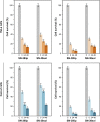
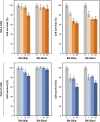
Results: Microfluidic, stable, and controlled sized, negatively charged liposomes, with high SN-38 incorporation efficiency into egg yolk phosphatidylcholine (EPC)/L-α-dioleoyl-phospathidylserine (DOPS) (9:1) vesicles (SN-38lip), were prepared. A lyophilized powder of SN-38lip, easily reconstitutable while retaining physicochemical parameters, was finally obtained. The efficacy of SN-38lip was assessed by in vitro studies with two tumor cell lines (HeLa and Caco-2) and compared with that of SN-38sol. It demonstrated the highest uptake of SN-38lip, in accordance with its highest cytotoxicity effect, in comparison with that of SN-38sol. In addition, different cell-cycle alterations were induced in both cell lines by the liposomal formulation.
Conclusion: The results highlight the potential usefulness of the procured SN-38 liposomal formulation and provide the basis for conducting in vivo studies that allow the development of alternative strategies for colorectal cancer treatment.
Keywords: SN-38; cell-cycle analysis; cytotoxicity; drug delivery; drug uptake; microfluidic liposomes.
Conflict of interest statement
Disclosure The authors report no conflicts of interest in this work.
Figures




Similar articles
-
Development and characterization of a novel liposome-based formulation of SN-38.Int J Pharm. 2004 Feb 11;270(1-2):93-107. doi: 10.1016/j.ijpharm.2003.10.015. Int J Pharm. 2004. PMID: 14726126
-
Formulation and in vitro characterization of thermosensitive liposomes for the delivery of irinotecan.J Pharm Sci. 2014 Oct;103(10):3127-38. doi: 10.1002/jps.24097. Epub 2014 Aug 4. J Pharm Sci. 2014. PMID: 25091422
-
Improved selectivity and cytotoxic effects of irinotecan via liposomal delivery: A comparative study on Hs68 and HeLa cells.Eur J Pharm Sci. 2017 Nov 15;109:65-77. doi: 10.1016/j.ejps.2017.07.024. Epub 2017 Jul 19. Eur J Pharm Sci. 2017. PMID: 28735042
-
Clinical pharmacokinetics of irinotecan.Clin Pharmacokinet. 1997 Oct;33(4):245-59. doi: 10.2165/00003088-199733040-00001. Clin Pharmacokinet. 1997. PMID: 9342501 Review.
-
Preclinical evaluation of CPT-11 and its active metabolite SN-38.Semin Oncol. 1996 Feb;23(1 Suppl 3):11-20. Semin Oncol. 1996. PMID: 8633248 Review.
Cited by
-
pH-responsive and folate-coated liposomes encapsulating irinotecan as an alternative to improve efficacy of colorectal cancer treatment.Biomed Pharmacother. 2021 Dec;144:112317. doi: 10.1016/j.biopha.2021.112317. Epub 2021 Oct 8. Biomed Pharmacother. 2021. PMID: 34634556 Free PMC article.
-
Liposomal Formulation of an Organogold Complex Enhancing Its Activity as Antimelanoma Agent-In Vitro and In Vivo Studies.Pharmaceutics. 2024 Dec 6;16(12):1566. doi: 10.3390/pharmaceutics16121566. Pharmaceutics. 2024. PMID: 39771545 Free PMC article.
-
Liposome-Embedding Silicon Microparticle for Oxaliplatin Delivery in Tumor Chemotherapy.Pharmaceutics. 2020 Jun 17;12(6):559. doi: 10.3390/pharmaceutics12060559. Pharmaceutics. 2020. PMID: 32560359 Free PMC article.
-
Carbon dots conjugated to SN38 for improved colorectal anticancer therapy.Mater Today Bio. 2022 Sep 1;16:100286. doi: 10.1016/j.mtbio.2022.100286. eCollection 2022 Dec. Mater Today Bio. 2022. PMID: 36186846 Free PMC article.
-
Current Landscape in Organic Nanosized Materials Advances for Improved Management of Colorectal Cancer Patients.Materials (Basel). 2021 May 8;14(9):2440. doi: 10.3390/ma14092440. Materials (Basel). 2021. PMID: 34066710 Free PMC article. Review.
References
-
- Stewart JC. Colorimetric determination of phospholipids with ammonium ferrothiocyanate. Anal Biochem. 1980;104(1):10–14. - PubMed
-
- Artursson P, Palm K, Luthman K. Caco-2 monolayers in experimental and theoretical predictions of drug transport. Adv Drug Deliv Rev. 2001;46(1–3):27–43. - PubMed
-
- Ferreira D, Adega F, Chaves R. The importance of cancer cell lines as in vitro models in cancer methylome analysis and anticancer drugs testing. In: López-Camarillo C, Aréchaga-Ocampo E, editors. Oncogenomics and Cancer Proteomics-Novel Approaches in Biomarkers Discovery and Therapeutic Targets in Cancer. InTechOpen; 2013. pp. 139–166. Available from: https://cdn.intechopen.com/pdfs-wm/43632.pdf.
-
- Mosmann T. Rapid calorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods. 1983;65(1–2):55–63. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

