Probing Synaptic Transmission and Behavior in Drosophila with Optogenetics: A Laboratory Exercise
- PMID: 30254546
- PMCID: PMC6153003
Probing Synaptic Transmission and Behavior in Drosophila with Optogenetics: A Laboratory Exercise
Abstract
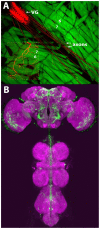
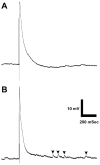
Optogenetics is possibly the most revolutionary advance in neuroscience research techniques within the last decade. Here, we describe lab modules, presented at a workshop for undergraduate neuroscience educators, using optogenetic control of neurons in the fruit fly Drosophila melanogaster. Drosophila is a genetically accessible model system that combines behavioral and neurophysiological complexity, ease of use, and high research relevance. One lab module utilized two transgenic Drosophila strains, each activating specific circuits underlying startle behavior and backwards locomotion, respectively. The red-shifted channelrhodopsin, CsChrimson, was expressed in neurons sharing a common transcriptional profile, with the expression pattern further refined by the use of a Split GAL4 intersectional activation system. Another set of strains was used to investigate synaptic transmission at the larval neuromuscular junction. These expressed Channelrhodopsin 2 (ChR2) in glutamatergic neurons, including the motor neurons. The first strain expressed ChR2 in a wild type background, while the second contained the SNAP-25ts mutant allele, which confers heightened evoked potential amplitude and greatly increased spontaneous vesicle release frequency at the larval neuromuscular junction. These modules introduced educators and students to the use of optogenetic stimulation to control behavior and evoked release at a model synapse, and establish a basis for students to explore neurophysiology using this technique, through recapitulating classic experiments and conducting independent research.
Keywords: Drosophila; SNAP-25; behavior; electrophysiology; neuromuscular junction; optogenetics.
Figures

References
-
- Boyden ES, Zhang F, Bamberg E, Nagel G, Deisseroth K. Millisecond-timescale, genetically targeted optical control of neural activity. Nat Neurosci. 2005;8:1263–1268. - PubMed
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous
