Physicochemical Factors That Favor Conjugation of an Antibiotic Resistant Plasmid in Non-growing Bacterial Cultures in the Absence and Presence of Antibiotics
- PMID: 30254617
- PMCID: PMC6141735
- DOI: 10.3389/fmicb.2018.02122
Physicochemical Factors That Favor Conjugation of an Antibiotic Resistant Plasmid in Non-growing Bacterial Cultures in the Absence and Presence of Antibiotics
Abstract
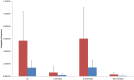
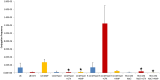
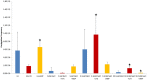
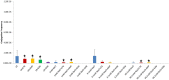
Horizontal gene transfer (HGT) of antibiotic resistance genes has received increased scrutiny from the scientific community in recent years owing to the public health threat associated with antibiotic resistant bacteria. Most studies have examined HGT in growing cultures. We examined conjugation in growing and non-growing cultures of E. coli using a conjugative multi antibiotic and metal resistant plasmid to determine physiochemical parameters that favor horizontal gene transfer. The conjugation frequency in growing and non-growing cultures was generally greater under shaken than non-shaken conditions, presumably due to increased frequency of cell collisions. Non-growing cultures in 9.1 mM NaCl had a similar conjugation frequency to that of growing cultures in Luria-Bertaini broth, whereas those in 1 mM or 90.1 mM NaCl were much lower. This salinity effect on conjugation was attributed to differences in cell-cell interactions and conformational changes in cell surface macromolecules. In the presence of antibiotics, the conjugation frequencies of growing cultures did not increase, but in non-growing cultures of 9.1 mM NaCl supplemented with Cefotaxime the conjugation frequency was as much as nine times greater than that of growing cultures. The mechanism responsible for the increased conjugation in non-growing bacteria was attributed to the likely lack of penicillin-binding protein 3 (the target of Cefotaxime), in non-growing cells that enabled Cefotaxime to interact with the plasmid and induce conjugation. Our results suggests that more attention may be owed to HGT in non-growing bacteria as most bacteria in the environment are likely not growing and the proposed mechanism for increased conjugation may not be unique to the bacteria/plasmid system we studied.
Keywords: antibiotic resistance; conjugation; horizontal gene transfer; non-growing bacteria; salinity; β-lactam antibiotics.
Figures

Similar articles
-
The Conjugation Window in an Escherichia coli K-12 Strain with an IncFII Plasmid.Appl Environ Microbiol. 2020 Aug 18;86(17):e00948-20. doi: 10.1128/AEM.00948-20. Print 2020 Aug 18. Appl Environ Microbiol. 2020. PMID: 32591383 Free PMC article.
-
Treatment with Cefotaxime Affects Expression of Conjugation Associated Proteins and Conjugation Transfer Frequency of an IncI1 Plasmid in Escherichia coli.Front Microbiol. 2017 Nov 29;8:2365. doi: 10.3389/fmicb.2017.02365. eCollection 2017. Front Microbiol. 2017. PMID: 29238335 Free PMC article.
-
Horizontal transfer of antibiotic resistance genes on abiotic touch surfaces: implications for public health.mBio. 2012 Nov 27;3(6):e00489-12. doi: 10.1128/mBio.00489-12. mBio. 2012. PMID: 23188508 Free PMC article.
-
Correlation between Exogenous Compounds and the Horizontal Transfer of Plasmid-Borne Antibiotic Resistance Genes.Microorganisms. 2020 Aug 8;8(8):1211. doi: 10.3390/microorganisms8081211. Microorganisms. 2020. PMID: 32784449 Free PMC article. Review.
-
Factors and Mechanisms Influencing Conjugation In Vivo in the Gastrointestinal Tract Environment: A Review.Int J Mol Sci. 2023 Mar 21;24(6):5919. doi: 10.3390/ijms24065919. Int J Mol Sci. 2023. PMID: 36982992 Free PMC article. Review.
Cited by
-
Influence of CO2 and Dust on the Survival of Non-Resistant and Multi-Resistant Airborne E. coli Strains.Antibiotics (Basel). 2024 Jun 14;13(6):558. doi: 10.3390/antibiotics13060558. Antibiotics (Basel). 2024. PMID: 38927224 Free PMC article.
-
Nutrition Related Stress Factors Reduce the Transfer of Extended-Spectrum Beta-Lactamase Resistance Genes between an Escherichia coli Donor and a Salmonella Typhimurium Recipient In Vitro.Biomolecules. 2019 Jul 31;9(8):324. doi: 10.3390/biom9080324. Biomolecules. 2019. PMID: 31370208 Free PMC article.
-
Predictive biology: modelling, understanding and harnessing microbial complexity.Nat Rev Microbiol. 2020 Sep;18(9):507-520. doi: 10.1038/s41579-020-0372-5. Epub 2020 May 29. Nat Rev Microbiol. 2020. PMID: 32472051 Review.
-
The impact of treatment strategies on the epidemiological dynamics of plasmid-conferred antibiotic resistance.Proc Natl Acad Sci U S A. 2024 Dec 24;121(52):e2406818121. doi: 10.1073/pnas.2406818121. Epub 2024 Dec 17. Proc Natl Acad Sci U S A. 2024. PMID: 39689176 Free PMC article.
-
Interference with Bacterial Conjugation and Natural Alternatives to Antibiotics: Bridging a Gap.Antibiotics (Basel). 2023 Jun 29;12(7):1127. doi: 10.3390/antibiotics12071127. Antibiotics (Basel). 2023. PMID: 37508224 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Other Literature Sources

