Assessment of Neutrophil Chemotaxis Upon G-CSF Treatment of Healthy Stem Cell Donors and in Allogeneic Transplant Recipients
- PMID: 30254629
- PMCID: PMC6141688
- DOI: 10.3389/fimmu.2018.01968
Assessment of Neutrophil Chemotaxis Upon G-CSF Treatment of Healthy Stem Cell Donors and in Allogeneic Transplant Recipients
Abstract
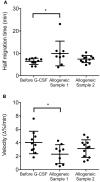
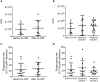
Neutrophils are crucial for the human innate immunity and constitute the majority of leukocytes in circulation. Thus, blood neutrophil counts serve as a measure for the immune system's functionality. Hematological patients often have low neutrophil counts due to disease or chemotherapy. To increase neutrophil counts and thereby preventing infections in high-risk patients, recombinant G-CSF is widely used as adjunct therapy to stimulate the maturation of neutrophils. In addition, G-CSF is utilized to recruit stem cells (SCs) into the peripheral blood of SC donors. Still, the actual functionality of neutrophils resulting from G-CSF treatment remains insufficiently understood. We tested the ex vivo functionality of neutrophils isolated from blood of G-CSF-treated healthy SC donors. We quantified chemotaxis, oxidative burst, and phagocytosis before and after treatment and detected significantly reduced chemotactic activity upon G-CSF treatment. Similarly, in vitro treatment of previously untreated neutrophils with G-CSF led to reduced chemotactic activity. In addition, we revealed that this effect persists in the allogeneic SC recipients up to 4 weeks after neutrophil engraftment. Our data indicates that neutrophil quantity, as a sole measure of immunocompetence in high-risk patients should be considered cautiously as neutrophil functionality might be affected by the primary treatment.
Keywords: allogeneic transplant; chemotaxis; granulocyte colony stimulating factor (G-CSF); hematopoietic stem cell donor; neutrophil.
Figures



Similar articles
-
Biosimilar Filgrastim (Tevagrastim, XMO2) for Allogeneic Hematopoietic Stem Cell Mobilization and Transplantation in Patients with Acute Myelogenous Leukemia/Myelodysplastic Syndromes.Biol Blood Marrow Transplant. 2016 Feb;22(2):277-283. doi: 10.1016/j.bbmt.2015.08.033. Epub 2015 Sep 4. Biol Blood Marrow Transplant. 2016. PMID: 26343949
-
In vitro and in vivo effects of granulocyte colony-stimulating factor on neutrophils in glycogen storage disease type 1B: granulocyte colony-stimulating factor therapy corrects the neutropenia and the defects in respiratory burst activity and Ca2+ mobilization.Pediatr Res. 1994 Jan;35(1):84-90. doi: 10.1203/00006450-199401000-00017. Pediatr Res. 1994. PMID: 7510873
-
Glycosylated and nonglycosylated recombinant human granulocyte colony-stimulating factor differently modifies actin polymerization in neutrophils.Clin Ter. 2006 Jan-Feb;157(1):19-24. Clin Ter. 2006. PMID: 16669548
-
Lymphokines: enhancement by granulocyte-macrophage and granulocyte colony-stimulating factors of neonatal myeloid kinetics and functional activation of polymorphonuclear leukocytes.Rev Infect Dis. 1990 May-Jun;12 Suppl 4:S492-7. doi: 10.1093/clinids/12.supplement_4.s492. Rev Infect Dis. 1990. PMID: 1694596 Review.
-
[Collection of hematopoietic progenitor cells from healthy donors].Acta Med Croatica. 2009 Jun;63(3):237-44. Acta Med Croatica. 2009. PMID: 19827352 Review. Croatian.
Cited by
-
Cytokine Augmentation Reverses Transplant Recipient Neutrophil Dysfunction Against the Human Fungal Pathogen Candida albicans.J Infect Dis. 2021 Sep 1;224(5):894-902. doi: 10.1093/infdis/jiab009. J Infect Dis. 2021. PMID: 33417688 Free PMC article.
-
Cellular sentinels: empowering survival and immune defense in hematopoietic stem cell transplantation through mesenchymal stem cells and T lymphocytes.BMC Med. 2025 Mar 18;23(1):164. doi: 10.1186/s12916-025-03987-2. BMC Med. 2025. PMID: 40102849 Free PMC article.
-
The impact of aging on neutrophil functions and the contribution to periodontitis.Int J Oral Sci. 2025 Jan 16;17(1):10. doi: 10.1038/s41368-024-00332-w. Int J Oral Sci. 2025. PMID: 39819982 Free PMC article. Review.
-
Uncovering the multifaceted roles played by neutrophils in allogeneic hematopoietic stem cell transplantation.Cell Mol Immunol. 2021 Apr;18(4):905-918. doi: 10.1038/s41423-020-00581-9. Epub 2020 Nov 17. Cell Mol Immunol. 2021. PMID: 33203938 Free PMC article. Review.
-
Candida albicans induces neutrophil extracellular traps and leucotoxic hypercitrullination via candidalysin.EMBO Rep. 2023 Nov 6;24(11):e57571. doi: 10.15252/embr.202357571. Epub 2023 Oct 5. EMBO Rep. 2023. PMID: 37795769 Free PMC article.
References
-
- Mcclatchey KD. Clinical Laboratory Medicine. Philadelphia, PA: Lippincott Williams and Wilkins; (2002).
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

