How Does the Sweet Violet (Viola odorata L.) Fight Pathogens and Pests - Cyclotides as a Comprehensive Plant Host Defense System
- PMID: 30254654
- PMCID: PMC6141879
- DOI: 10.3389/fpls.2018.01296
How Does the Sweet Violet (Viola odorata L.) Fight Pathogens and Pests - Cyclotides as a Comprehensive Plant Host Defense System
Abstract
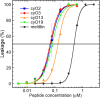
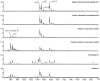
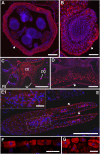
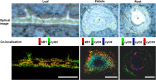
Cyclotides are cyclic plant polypeptides of 27-37 amino acid residues. They have been extensively studied in bioengineering and drug development contexts. However, less is known about the relevance of cyclotides for the plants producing them. The anti-insect larvae effects of kB1 and antibacterial activity of cyO2 suggest that cyclotides are a part of plant host defense. The sweet violet (Viola odorata L.) produces a wide array of cyclotides, including kB1 (kalata B1) and cyO2 (cycloviolacin O2), with distinct presumed biological roles. Here, we evaluate V. odorata cyclotides' potency against plant pathogens and their mode of action using bioassays, liposome experiments and immunogold labeling for transmission electron microscopy (TEM). We explore the link between the biological activity and distribution in plant generative, vegetative tissues and seeds, depicted by immunohistochemistry and matrix assisted laser desorption ionization mass spectrometry imaging (MALDI-MSI). Cyclotides cyO2, cyO3, cyO13, and cyO19 are shown to have potent activity against model fungal plant pathogens (Fusarium oxysporum, F. graminearum, F. culmorum, Mycosphaerella fragariae, Botrytis cinerea) and fungi isolated from violets (Colletotrichum utrechtense and Alternaria alternata), with minimal inhibitory concentrations (MICs) ranging from 0.8 μM to 25 μM. Inhibition of phytopathogenic bacteria - Pseudomonas syringae pv. syringae, Dickeya dadantii and Pectobacterium atrosepticum - is also observed with MIC = 25-100 μM. A membrane-disrupting antifungal mode of action is shown. Finding cyO2 inside the fungal spore cells in TEM images may indicate that other, intracellular targets may be involved in the mechanism of toxicity. Fungi can not break down cyclotides in the course of days. varv A (kalata S) and kB1 show little potency against pathogenic fungi when compared with the tested cycloviolacins. cyO2, cyO3, cyO19 and kB1 are differentially distributed and found in tissues vulnerable to pathogen (epidermis, rizodermis, vascular bundles, protodermis, procambium, ovary walls, outer integuments) and pest (ground tissues of leaf and petiole) attacks, respectively, indicating a link between the cyclotides' sites of accumulation and biological role. Cyclotides emerge as a comprehensive defense system in V. odorata, in which different types of peptides have specific targets that determine their distribution in plant tissues.
Keywords: MALDI-MSI; Violaceae; antifungal defense; antimicrobial peptide; cyclotides; immunohistochemistry; plant host defense.
Figures






Similar articles
-
Immunolocalization of cyclotides in plant cells, tissues and organ supports their role in host defense.Planta. 2016 Nov;244(5):1029-1040. doi: 10.1007/s00425-016-2562-y. Epub 2016 Jul 9. Planta. 2016. PMID: 27394154 Free PMC article.
-
Behavioral and physiological effects of Viola spp. cyclotides on Myzus persicae (Sulz.).J Insect Physiol. 2020 Apr;122:104025. doi: 10.1016/j.jinsphys.2020.104025. Epub 2020 Feb 12. J Insect Physiol. 2020. PMID: 32059835
-
The cyclotide cycloviolacin O2 from Viola odorata has potent bactericidal activity against Gram-negative bacteria.J Antimicrob Chemother. 2010 Sep;65(9):1964-71. doi: 10.1093/jac/dkq220. Epub 2010 Jun 17. J Antimicrob Chemother. 2010. PMID: 20558471
-
Host-defense activities of cyclotides.Toxins (Basel). 2012 Feb;4(2):139-56. doi: 10.3390/toxins4020139. Epub 2012 Feb 15. Toxins (Basel). 2012. PMID: 22474571 Free PMC article. Review.
-
Novel strategies for isolation and characterization of cyclotides: the discovery of bioactive macrocyclic plant polypeptides in the Violaceae.Curr Protein Pept Sci. 2004 Oct;5(5):317-29. doi: 10.2174/1389203043379495. Curr Protein Pept Sci. 2004. PMID: 15544528 Review.
Cited by
-
In vitro Inhibition of HIV-1 by Cyclotide-Enriched Extracts of Viola tricolor.Front Pharmacol. 2022 May 27;13:888961. doi: 10.3389/fphar.2022.888961. eCollection 2022. Front Pharmacol. 2022. PMID: 35712712 Free PMC article.
-
Kalata B1 Enhances Temozolomide Toxicity to Glioblastoma Cells.Biomedicines. 2024 Sep 28;12(10):2216. doi: 10.3390/biomedicines12102216. Biomedicines. 2024. PMID: 39457529 Free PMC article.
-
Isolation of antimicrobial peptides from different plant sources: Does a general extraction method exist?Plant Methods. 2020 Oct 23;16:143. doi: 10.1186/s13007-020-00687-1. eCollection 2020. Plant Methods. 2020. PMID: 33110440 Free PMC article. Review.
-
Nutritional and Pharmaceutical Applications of Under-Explored Knottin Peptide-Rich Phytomedicines.Plants (Basel). 2022 Nov 28;11(23):3271. doi: 10.3390/plants11233271. Plants (Basel). 2022. PMID: 36501311 Free PMC article. Review.
-
Insights of the Neofusicoccum parvum-Liquidambar styraciflua Interaction and Identification of New Cysteine-Rich Proteins in Both Species.J Fungi (Basel). 2021 Nov 30;7(12):1027. doi: 10.3390/jof7121027. J Fungi (Basel). 2021. PMID: 34947009 Free PMC article.
References
-
- Bell K. S., Sebaihia M., Pritchard L., Holden M. T., Hyman L. J., Holeva M. C., et al. (2004). Genome sequence of the enterobacterial phytopathogen Erwinia carotovora subsp. atroseptica and characterization of virulence factors. Proc. Natl Acad. Sci. U.S.A. 101 11105–11110. 10.1073/pnas.0402424101 - DOI - PMC - PubMed
-
- Broekaert W. F., Terras F., Cammue B., Vandedeyden J. (1990). An automated quantitative assay for fungal growth inhibition. FEMS Microbiol. Lett. 69 55–60. 10.1016/0378-1097(90)90412-J - DOI
LinkOut - more resources
Full Text Sources
Other Literature Sources

