Botulinum Neurotoxin Application to the Severed Femoral Nerve Modulates Spinal Synaptic Responses to Axotomy and Enhances Motor Recovery in Rats
- PMID: 30254669
- PMCID: PMC6145158
- DOI: 10.1155/2018/7975013
Botulinum Neurotoxin Application to the Severed Femoral Nerve Modulates Spinal Synaptic Responses to Axotomy and Enhances Motor Recovery in Rats
Abstract
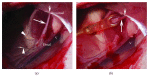
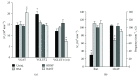
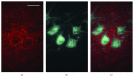
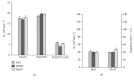
Botulinum neurotoxin A (BoNT) and brain-derived neurotrophic factor (BDNF) are known for their ability to influence synaptic inputs to neurons. Here, we tested if these drugs can modulate the deafferentation of motoneurons following nerve section/suture and, as a consequence, modify the outcome of peripheral nerve regeneration. We applied drug solutions to the proximal stump of the freshly cut femoral nerve of adult rats to achieve drug uptake and transport to the neuronal perikarya. The most marked effect of this application was a significant reduction of the axotomy-induced loss of perisomatic cholinergic terminals by BoNT at one week and two months post injury. The attenuation of the synaptic deficit was associated with enhanced motor recovery of the rats 2-20 weeks after injury. Although BDNF also reduced cholinergic terminal loss at 1 week, it had no effect on this parameter at two months and no effect on functional recovery. These findings strengthen the idea that persistent partial deafferentation of axotomized motoneurons may have a significant negative impact on functional outcome after nerve injury. Intraneural application of drugs may be a promising way to modify deafferentation and, thus, elucidate relationships between synaptic plasticity and restoration of function.
Figures

Similar articles
-
A dose-dependent facilitation and inhibition of peripheral nerve regeneration by brain-derived neurotrophic factor.Eur J Neurosci. 2002 Feb;15(4):613-26. doi: 10.1046/j.1460-9568.2002.01891.x. Eur J Neurosci. 2002. PMID: 11886442
-
Prolonged target deprivation reduces the capacity of injured motoneurons to regenerate.Neurosurgery. 2007 Apr;60(4):723-32; discussion 732-3. doi: 10.1227/01.NEU.0000255412.63184.CC. Neurosurgery. 2007. PMID: 17415210
-
Neuroprotective effects of mesenchymal stem cells on spinal motoneurons following ventral root axotomy: synapse stability and axonal regeneration.Neuroscience. 2013 Oct 10;250:715-32. doi: 10.1016/j.neuroscience.2013.07.043. Epub 2013 Jul 27. Neuroscience. 2013. PMID: 23896572
-
The role of neurotrophic factors in nerve regeneration.Neurosurg Focus. 2009 Feb;26(2):E3. doi: 10.3171/FOC.2009.26.2.E3. Neurosurg Focus. 2009. PMID: 19228105 Review.
-
Putative roles of soluble trophic factors in facial nerve regeneration, target reinnervation, and recovery of vibrissal whisking.Exp Neurol. 2018 Feb;300:100-110. doi: 10.1016/j.expneurol.2017.10.029. Epub 2017 Nov 8. Exp Neurol. 2018. PMID: 29104116 Review.
Cited by
-
The course of recovery of locomotor function over a 10-week observation period in a rat model of femoral nerve resection and autograft repair.Brain Behav. 2020 Apr;10(4):e01580. doi: 10.1002/brb3.1580. Epub 2020 Feb 25. Brain Behav. 2020. PMID: 32097542 Free PMC article.
-
Botulinum Toxin and Neuronal Regeneration after Traumatic Injury of Central and Peripheral Nervous System.Toxins (Basel). 2020 Jul 2;12(7):434. doi: 10.3390/toxins12070434. Toxins (Basel). 2020. PMID: 32630737 Free PMC article. Review.
-
The Neuroregenerative Effects of IncobotulinumtoxinA (Inco/A) in a Nerve Lesion Model of the Rat.Int J Mol Sci. 2025 Aug 2;26(15):7482. doi: 10.3390/ijms26157482. Int J Mol Sci. 2025. PMID: 40806609 Free PMC article.
-
Preclinical Evidence for the Role of Botulinum Neurotoxin A (BoNT/A) in the Treatment of Peripheral Nerve Injury.Microorganisms. 2022 Apr 24;10(5):886. doi: 10.3390/microorganisms10050886. Microorganisms. 2022. PMID: 35630331 Free PMC article. Review.
-
Mice lacking perforin have improved regeneration of the injured femoral nerve.Neural Regen Res. 2022 Aug;17(8):1802-1808. doi: 10.4103/1673-5374.332152. Neural Regen Res. 2022. PMID: 35017441 Free PMC article.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

