Immune mechanisms in the different phases of acute tubular necrosis
- PMID: 30254843
- PMCID: PMC6147180
- DOI: 10.23876/j.krcp.2018.37.3.185
Immune mechanisms in the different phases of acute tubular necrosis
Abstract
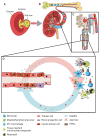
Acute kidney injury is a clinical syndrome that can be caused by numerous diseases including acute tubular necrosis (ATN). ATN evolves in several phases, all of which are accompanied by different immune mechanisms as an integral component of the disease process. In the early injury phase, regulated necrosis, damage-associated molecular patterns, danger sensing, and neutrophil-driven sterile inflammation enhance each other and contribute to the crescendo of necroinflammation and tissue injury. In the late injury phase, renal dysfunction becomes clinically apparent, and M1 macrophage-driven sterile inflammation contributes to ongoing necroinflammation and renal dysfunction. In the recovery phase, M2-macrophages and anti-inflammatory mediators counteract the inflammatory process, and compensatory remnant nephron and cell hypertrophy promote an early functional recovery of renal function, while some tubules are still badly injured and necrotic material is removed by phagocytes. The resolution of inflammation is required to promote the intrinsic regenerative capacity of tubules to replace at least some of the necrotic cells. Several immune mechanisms support this wound-healing-like re-epithelialization process. Similar to wound healing, this response is associated with mesenchymal healing, with a profound immune cell contribution in terms of collagen production and secretion of profibrotic mediators. These and numerous other factors determine whether, in the chronic phase, persistent loss of nephrons and hyperfunction of remnant nephrons will result in stable renal function or progress to decline of renal function such as progressive chronic kidney disease.
Keywords: Acute kidney injury; Extracellular traps; Injury; Necroptosis; Therapeutics.
Conflict of interest statement
Conflicts of interest All authors have no conflicts of interest to declare.
Figures

Similar articles
-
Targeting Inflammation in So-Called Acute Kidney Injury.Semin Nephrol. 2016 Jan;36(1):17-30. doi: 10.1016/j.semnephrol.2016.01.006. Semin Nephrol. 2016. PMID: 27085732 Review.
-
How Kidney Cell Death Induces Renal Necroinflammation.Semin Nephrol. 2016 May;36(3):162-73. doi: 10.1016/j.semnephrol.2016.03.004. Semin Nephrol. 2016. PMID: 27339382 Review.
-
Mechanisms and Models of Kidney Tubular Necrosis and Nephron Loss.J Am Soc Nephrol. 2022 Mar;33(3):472-486. doi: 10.1681/ASN.2021101293. Epub 2022 Jan 12. J Am Soc Nephrol. 2022. PMID: 35022311 Free PMC article. Review.
-
Histones and Neutrophil Extracellular Traps Enhance Tubular Necrosis and Remote Organ Injury in Ischemic AKI.J Am Soc Nephrol. 2017 Jun;28(6):1753-1768. doi: 10.1681/ASN.2016080925. Epub 2017 Jan 10. J Am Soc Nephrol. 2017. PMID: 28073931 Free PMC article.
-
Extracellular traps in kidney disease.Kidney Int. 2018 Dec;94(6):1087-1098. doi: 10.1016/j.kint.2018.08.035. Kidney Int. 2018. PMID: 30466565 Review.
Cited by
-
Role of T cells in ischemic acute kidney injury and repair.Korean J Intern Med. 2022 May;37(3):534-550. doi: 10.3904/kjim.2021.526. Epub 2022 Apr 28. Korean J Intern Med. 2022. PMID: 35508946 Free PMC article. Review.
-
RNA-Seq identifies condition-specific biological signatures of ischemia-reperfusion injury in the human kidney.BMC Nephrol. 2020 Sep 25;21(Suppl 1):398. doi: 10.1186/s12882-020-02025-y. BMC Nephrol. 2020. PMID: 32977749 Free PMC article.
-
The Role of Immune-Related miRNAs in the Pathology of Kidney Transplantation.Biomolecules. 2021 Aug 12;11(8):1198. doi: 10.3390/biom11081198. Biomolecules. 2021. PMID: 34439863 Free PMC article. Review.
-
Rhabdomyolysis-Induced AKI Was Ameliorated in NLRP3 KO Mice via Alleviation of Mitochondrial Lipid Peroxidation in Renal Tubular Cells.Int J Mol Sci. 2020 Nov 13;21(22):8564. doi: 10.3390/ijms21228564. Int J Mol Sci. 2020. PMID: 33202867 Free PMC article.
-
Toll-Like Receptors in Acute Kidney Injury.Int J Mol Sci. 2021 Jan 15;22(2):816. doi: 10.3390/ijms22020816. Int J Mol Sci. 2021. PMID: 33467524 Free PMC article. Review.
References
-
- Khwaja A. KDIGO clinical practice guidelines for acute kidney injury. Nephron Clin Pract. 2012;120:c179–c184. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

