Efficacy of Hormonal Therapies for Decreasing Uterine Volume in Patients with Adenomyosis
- PMID: 30254953
- PMCID: PMC6135169
- DOI: 10.4103/GMIT.GMIT_35_18
Efficacy of Hormonal Therapies for Decreasing Uterine Volume in Patients with Adenomyosis
Abstract
Study objective: The aim of this study is to evaluate the efficacy of hormonal therapies for inhibiting an increase in uterine volume in patients with adenomyosis.
Design: This was retrospective cohort study.
Setting: This study was conducted at Nippon Medical School Musashikosugi Hospital.
Patients: A total of 28 women diagnosed with adenomyosis using magnetic resonance imaging.
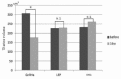
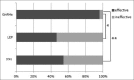
Methods: After providing informed consent, patients were treated with gonadotropin-releasing hormone agonist (GnRHa group), a low-dose estrogen and progestin combination (LEP group), or dienogest (DNG group) for ≥16 weeks. Uterine volume was assessed using the formula for an ovoid; uterine volumes before and after 16 weeks of treatment were compared. A <5% increase in uterine volume at 16 weeks was considered to reflect inhibition of uterine volume increase and efficacy of the medication. We compared the efficacy rate among the groups.
Results: In the GnRHa group, a significant reduction in uterine volume was noted, from 307.4 ± 230.1 to 177.9 ± 142.1 cm3 (P < 0.001). In the LEP and the DNG groups, there was no significant change (LEP: 226.7 ± 116.6 cm3 pre-treatment and 230.5 ± 128.6 cm3 post-treatment, P = 0.85; DNG: 232.6 ± 117.8 cm3 pre-treatment and 262.1 ± 136.8 cm3 post-treatment, P = 0.37). The number of responders (efficacy rate) in the GnRHa group, LEP group, and DNG group was 25/26 (96.2%), 7/15 (46.7%), and 6/11 (54.5%), respectively. The efficacy rate of GnRHa therapy was significantly higher than that of LEP or DNG therapy (P < 0.001 and P = 0.005, respectively).
Conclusion: We conclude that the efficacy of GnRHa in reducing uterine volume should be considered when prescribing hormone therapy for adenomyosis.
Keywords: Adenomyosis; dienogest; ethinyl estradiol; ferrous fumarate drug combination; gonadotropin-releasing hormone; norethindrone acetate.
Conflict of interest statement
There are no conflicts of interest.
Figures
References
-
- Benagiano G, Brosens I, Habiba M. Adenomyosis: A life-cycle approach. Reprod Biomed Online. 2015;30:220–32. - PubMed
-
- Pontis A, D’Alterio MN, Pirarba S, de Angelis C, Tinelli R, Angioni S, et al. Adenomyosis: A systematic review of medical treatment. Gynecol Endocrinol. 2016;32:696–700. - PubMed
-
- Shiota M, Kotani Y, Umemoto M, Tobiume T, Tsuritani M, Shimaoka M, et al. Deep-vein thrombosis is associated with large uterine fibroids. Tohoku J Exp Med. 2011;224:87–9. - PubMed
-
- Akira S, Iwasaki N, Ichikawa M, Mine K, Kuwabara Y, Takeshita T, et al. Successful long-term management of adenomyosis associated with deep thrombosis by low-dose gonadotropin-releasing hormone agonist therapy. Clin Exp Obstet Gynecol. 2009;36:123–5. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

