Metabolic Mechanisms of Exercise-Induced Cardiac Remodeling
- PMID: 30255026
- PMCID: PMC6141631
- DOI: 10.3389/fcvm.2018.00127
Metabolic Mechanisms of Exercise-Induced Cardiac Remodeling
Abstract
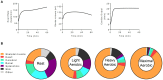
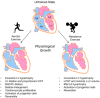
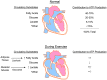
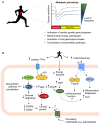
Exercise has a myriad of physiological benefits that derive in part from its ability to improve cardiometabolic health. The periodic metabolic stress imposed by regular exercise appears fundamental in driving cardiovascular tissue adaptation. However, different types, intensities, or durations of exercise elicit different levels of metabolic stress and may promote distinct types of tissue remodeling. In this review, we discuss how exercise affects cardiac structure and function and how exercise-induced changes in metabolism regulate cardiac adaptation. Current evidence suggests that exercise typically elicits an adaptive, beneficial form of cardiac remodeling that involves cardiomyocyte growth and proliferation; however, chronic levels of extreme exercise may increase the risk for pathological cardiac remodeling or sudden cardiac death. An emerging theme underpinning acute as well as chronic cardiac adaptations to exercise is metabolic periodicity, which appears important for regulating mitochondrial quality and function, for stimulating metabolism-mediated exercise gene programs and hypertrophic kinase activity, and for coordinating biosynthetic pathway activity. In addition, circulating metabolites liberated during exercise trigger physiological cardiac growth. Further understanding of how exercise-mediated changes in metabolism orchestrate cell signaling and gene expression could facilitate therapeutic strategies to maximize the benefits of exercise and improve cardiac health.
Keywords: cardiomyopathy; cell signaling; exercise; glucose; heart; hypertrophy; metabokine; mitochondria.
Figures
Similar articles
-
Exercise-Induced Changes in Glucose Metabolism Promote Physiological Cardiac Growth.Circulation. 2017 Nov 28;136(22):2144-2157. doi: 10.1161/CIRCULATIONAHA.117.028274. Epub 2017 Aug 31. Circulation. 2017. PMID: 28860122 Free PMC article.
-
Coordinated Metabolic Responses Facilitate Cardiac Growth in Pregnancy and Exercise.Curr Heart Fail Rep. 2023 Oct;20(5):441-450. doi: 10.1007/s11897-023-00622-0. Epub 2023 Aug 15. Curr Heart Fail Rep. 2023. PMID: 37581772 Free PMC article. Review.
-
Metabolic Coordination of Physiological and Pathological Cardiac Remodeling.Circ Res. 2018 Jun 22;123(1):107-128. doi: 10.1161/CIRCRESAHA.118.312017. Circ Res. 2018. PMID: 29929976 Free PMC article. Review.
-
Energy Metabolism in Exercise-Induced Physiologic Cardiac Hypertrophy.Front Pharmacol. 2020 Jul 29;11:1133. doi: 10.3389/fphar.2020.01133. eCollection 2020. Front Pharmacol. 2020. PMID: 32848751 Free PMC article. Review.
-
The Non-cardiomyocyte Cells of the Heart. Their Possible Roles in Exercise-Induced Cardiac Regeneration and Remodeling.Adv Exp Med Biol. 2017;999:117-136. doi: 10.1007/978-981-10-4307-9_8. Adv Exp Med Biol. 2017. PMID: 29022261 Review.
Cited by
-
Metabolic signatures of pregnancy-induced cardiac growth.Am J Physiol Heart Circ Physiol. 2022 Jul 1;323(1):H146-H164. doi: 10.1152/ajpheart.00105.2022. Epub 2022 May 27. Am J Physiol Heart Circ Physiol. 2022. PMID: 35622533 Free PMC article.
-
Physical Exercise Promotes a Reduction in Cardiac Fibrosis in the Chronic Indeterminate Form of Experimental Chagas Disease.Front Immunol. 2021 Nov 4;12:712034. doi: 10.3389/fimmu.2021.712034. eCollection 2021. Front Immunol. 2021. PMID: 34804007 Free PMC article.
-
Lack of sex-specific differences in the associations between the dimensions of great vessels and exercise performance in amateur cyclists.PLoS One. 2024 Nov 4;19(11):e0313165. doi: 10.1371/journal.pone.0313165. eCollection 2024. PLoS One. 2024. PMID: 39495753 Free PMC article.
-
Lactate modulates cardiac gene expression in mice during acute physical exercise.Braz J Med Biol Res. 2022 Apr 27;55:e11820. doi: 10.1590/1414-431X2022e11820. eCollection 2022. Braz J Med Biol Res. 2022. PMID: 35588524 Free PMC article.
-
Mitochondrial Dysfunction in Cardiac Disease: The Fort Fell.Biomolecules. 2024 Nov 29;14(12):1534. doi: 10.3390/biom14121534. Biomolecules. 2024. PMID: 39766241 Free PMC article. Review.
References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

