Dynamic and Sex-Specific Changes in Gonadotropin-Releasing Hormone Neuron Activity and Excitability in a Mouse Model of Temporal Lobe Epilepsy
- PMID: 30255128
- PMCID: PMC6153338
- DOI: 10.1523/ENEURO.0273-18.2018
Dynamic and Sex-Specific Changes in Gonadotropin-Releasing Hormone Neuron Activity and Excitability in a Mouse Model of Temporal Lobe Epilepsy
Abstract
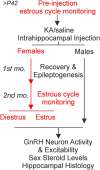
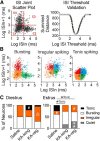
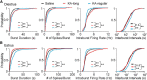
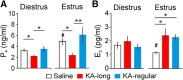
Reproductive endocrine disorders are prominent comorbidities of temporal lobe epilepsy (TLE) in both men and women. The neural mechanisms underlying these comorbidities remain unclear, but hypothalamic gonadotropin-releasing hormone (GnRH) neurons may be involved. Here, we report the first direct demonstrations of aberrant GnRH neuron function in an animal model of epilepsy. Recordings of GnRH neuron firing and excitability were made in acute mouse brain slices prepared two months after intrahippocampal injection of kainate (KA) or control saline, a well-established TLE model in which most females develop comorbid estrous cycle disruption. GnRH neurons from control females showed elevated firing and excitability on estrus compared with diestrus. By contrast, cells from KA-injected females that developed prolonged, disrupted estrous cycles (KA-long) showed the reverse pattern. Firing rates of cells from KA-injected females that maintained regular cycles (KA-regular) were not different from controls on diestrus, but were reduced on estrus. In KA-injected males, only GnRH neurons in the medial septum displayed elevated firing. In contrast to the diestrus versus estrus and sex-specific changes in firing, GnRH neuron intrinsic excitability was elevated in all KA-injected groups, indicating a role for afferent synaptic and neuromodulatory inputs in shaping overall changes in firing activity. Furthermore, KA-injected females showed cycle-stage-specific changes in circulating sex steroids on diestrus and estrus that also differed between KA-long and KA-regular groups. Together, these findings reveal that the effects of epilepsy on the neural control of reproduction are dynamic across the estrous cycle, distinct in association with comorbid estrous cycle disruption severity, and sex-specific.
Keywords: GnRH; excitability; hormone; patch clamp electrophysiology; temporal lobe epilepsy.
Figures





Similar articles
-
Increased GABA transmission to GnRH neurons after intrahippocampal kainic acid injection in mice is sex-specific and associated with estrous cycle disruption.Neurobiol Dis. 2022 Oct 1;172:105822. doi: 10.1016/j.nbd.2022.105822. Epub 2022 Jul 20. Neurobiol Dis. 2022. PMID: 35868435 Free PMC article.
-
Sex-Dependent Changes in Gonadotropin-Releasing Hormone Neuron Voltage-Gated Potassium Currents in a Mouse Model of Temporal Lobe Epilepsy.eNeuro. 2024 Oct 21;11(10):ENEURO.0324-24.2024. doi: 10.1523/ENEURO.0324-24.2024. Print 2024 Oct. eNeuro. 2024. PMID: 39375030 Free PMC article.
-
Epilepsy-associated increase in gonadotropin-releasing hormone neuron firing in diestrous female mice is independent of chronic seizure burden severity.Epilepsy Res. 2022 Aug;184:106948. doi: 10.1016/j.eplepsyres.2022.106948. Epub 2022 May 20. Epilepsy Res. 2022. PMID: 35690025 Free PMC article.
-
Understanding calcium homeostasis in postnatal gonadotropin-releasing hormone neurons using cell-specific Pericam transgenics.Cell Calcium. 2012 Mar-Apr;51(3-4):267-76. doi: 10.1016/j.ceca.2011.11.005. Epub 2011 Dec 15. Cell Calcium. 2012. PMID: 22177387 Review.
-
Glutamate regulation of GnRH neuron excitability.Brain Res. 2010 Dec 10;1364:35-43. doi: 10.1016/j.brainres.2010.08.071. Epub 2010 Aug 31. Brain Res. 2010. PMID: 20807514 Review.
Cited by
-
Estrous Cycle Monitoring in Mice with Rapid Data Visualization and Analysis.Bio Protoc. 2019 Sep 5;9(17):e3354. doi: 10.21769/BioProtoc.3354. Bio Protoc. 2019. PMID: 32695847 Free PMC article.
-
Female-specific pituitary gonadotrope dysregulation in mice with chronic focal epilepsy.Exp Neurol. 2023 Jun;364:114389. doi: 10.1016/j.expneurol.2023.114389. Epub 2023 Mar 28. Exp Neurol. 2023. PMID: 36990138 Free PMC article.
-
Evaluating the interplay between estrous cyclicity and flurothyl-induced seizure susceptibility in Scn2a K1422E mice.MicroPubl Biol. 2023 Dec 16;2023:10.17912/micropub.biology.000850. doi: 10.17912/micropub.biology.000850. eCollection 2023. MicroPubl Biol. 2023. PMID: 38162411 Free PMC article.
-
Sex and estrous cycle stage shape left-right asymmetry in chronic hippocampal seizures in mice.bioRxiv [Preprint]. 2023 Jan 21:2023.01.20.524965. doi: 10.1101/2023.01.20.524965. bioRxiv. 2023. Update in: eNeuro. 2023 Jun 5;10(6):ENEURO.0041-23.2023. doi: 10.1523/ENEURO.0041-23.2023. PMID: 36712086 Free PMC article. Updated. Preprint.
-
Sex on the Brain: Reproductive Comorbidities in Temporal Lobe Epilepsy.Epilepsy Curr. 2022 Nov 29;23(1):58-60. doi: 10.1177/15357597221135717. eCollection 2023 Jan-Feb. Epilepsy Curr. 2022. PMID: 36923330 Free PMC article. No abstract available.
References
-
- Amado D, Verreschi IT, Berzaghi MP, Cavalheiro EA (1987) Effects of intrahippocampal injection of kainic acid on estrous cycle in rats. Braz J Med Biol Res 20:829–832. - PubMed
-
- Amado D, Cavalheiro E, Bentivoglio M (1993) Epilepsy and hormonal regulation: the patterns of GnRH and galanin immunoreactivity in the hypothalamus of epileptic female rats. Epilepsy Res 14:149–159. - PubMed
-
- Bauer J, Jarre A, Klingmüller D, Elger CE (2000) Polycystic ovary syndrome in patients with focal epilepsy: a study in 93 women. Epilepsy Res 41:163–167. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
