Oleoylethanolamide and Palmitoylethanolamide Protect Cultured Cortical Neurons Against Hypoxia
- PMID: 30255158
- PMCID: PMC6148719
- DOI: 10.1089/can.2018.0013
Oleoylethanolamide and Palmitoylethanolamide Protect Cultured Cortical Neurons Against Hypoxia
Abstract
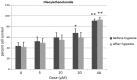
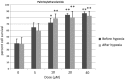
Introduction: Perinatal hypoxic-ischemic (HI) encephalopathy is defined as a neurological syndrome where the newborn suffers from acute ischemia and hypoxia during the perinatal period. New therapies are needed. The acylethanolamides, oleoylethanolamide (OEA) and palmitoylethanolamide (PEA), possess neuroprotective properties, and they could be effective against perinatal HI. These lipid mediators act through peroxisome proliferator-activated receptors subtype α (PPARα), or transient receptor potential vanilloid (TRPV), such as TRPV subtype 1 and 4. Materials and Methods: The objectives of this study were to discern: (1) the neuroprotective role of OEA and PEA in parietotemporal cortical neurons of newborn rats and mice subjected to hypoxia, and (2) the role of the receptors, PPARα, TRPV1, and TRPV4, in neuroprotective effects. Cell culture of cortical neurons and the lactate dehydrogenase assay was carried out. The role of receptors was discerned by using selective antagonist and agonist ligands, as well as knockout (KO) PPARα mice. Results: The findings indicate that OEA and PEA exert neuroprotective effects on cultured cortical neurons subjected to a hypoxic episode. These protective effects are not mediated by the receptors, PPARα, TRPV1, or TRPV4, because neither PPARα KO mice nor receptor ligands significantly modify OEA and PEA-induced effects. Blocking TRPV4 with RN1734 is neuroprotective per se, and cotreatment with OEA and PEA is able to enhance neuroprotective effects of the acylethanolamides. Since stimulating TRPV4 was devoid of effects on OEA and PEA-induced protective effects, effects of RN1734 cotreatment seem to be a consequence of additive actions. Conclusion: The lipid mediators, OEA and PEA, exert neuroprotective effects on cultured cortical neurons subjected to hypoxia. Coadministration of OEA or PEA, and the TRPV4 antagonist RN1734 is able to enhance neuroprotective effects. These in vitro results could be of utility for developing new therapeutic tools against perinatal HI.
Keywords: PPARα; TRPV4; hypoxic–ischemic; neuroprotection; oleoylethanolamide; palmitoylethanolamide.
Conflict of interest statement
The authors declare that there are no conflicts of interest.
Figures

Similar articles
-
Palmitoylethanolamide and other anandamide congeners. Proposed role in the diseased brain.Exp Neurol. 2010 Jul;224(1):48-55. doi: 10.1016/j.expneurol.2010.03.022. Epub 2010 Mar 29. Exp Neurol. 2010. PMID: 20353771 Review.
-
In vivo brain levels of acetylcholine and 5-hydroxytryptamine after oleoylethanolamide or palmitoylethanolamide administrations are mediated by PPARα engagement.Eur J Neurosci. 2021 Sep;54(6):5932-5950. doi: 10.1111/ejn.15409. Epub 2021 Aug 15. Eur J Neurosci. 2021. PMID: 34396611
-
Oleoylethanolamide Protects against Acute Ischemic Stroke by Promoting PPARα-Mediated Microglia/Macrophage M2 Polarization.Pharmaceuticals (Basel). 2023 Apr 20;16(4):621. doi: 10.3390/ph16040621. Pharmaceuticals (Basel). 2023. PMID: 37111378 Free PMC article.
-
Oleoylethanolamide and Palmitoylethanolamide Enhance IFNβ-Induced Apoptosis in Human Neuroblastoma SH-SY5Y Cells.Molecules. 2024 Apr 2;29(7):1592. doi: 10.3390/molecules29071592. Molecules. 2024. PMID: 38611871 Free PMC article.
-
Physiological role of peroxisome proliferator-activated receptors type α on dopamine systems.CNS Neurol Disord Drug Targets. 2013 Feb 1;12(1):70-7. doi: 10.2174/1871527311312010012. CNS Neurol Disord Drug Targets. 2013. PMID: 23394525 Review.
Cited by
-
N-Acylethanolamine-Hydrolyzing Acid Amidase Inhibition, but Not Fatty Acid Amide Hydrolase Inhibition, Prevents the Development of Experimental Autoimmune Encephalomyelitis in Mice.Neurotherapeutics. 2021 Jul;18(3):1815-1833. doi: 10.1007/s13311-021-01074-x. Epub 2021 Jul 7. Neurotherapeutics. 2021. PMID: 34235639 Free PMC article.
-
Opposite Effects of Neuroprotective Cannabinoids, Palmitoylethanolamide, and 2-Arachidonoylglycerol on Function and Morphology of Microglia.Front Neurosci. 2019 Nov 7;13:1180. doi: 10.3389/fnins.2019.01180. eCollection 2019. Front Neurosci. 2019. PMID: 31787870 Free PMC article.
-
Alterations of the endocannabinoid system in autism spectrum disorder: a systematic review and meta-analysis.Eur Arch Psychiatry Clin Neurosci. 2025 Jun 15. doi: 10.1007/s00406-025-02031-x. Online ahead of print. Eur Arch Psychiatry Clin Neurosci. 2025. PMID: 40518463
-
Amantadine Attenuated Hypoxia-Induced Mitochondrial Oxidative Neurotoxicity, Apoptosis, and Inflammation via the Inhibition of TRPM2 and TRPV4 Channels.Mol Neurobiol. 2022 Jun;59(6):3703-3720. doi: 10.1007/s12035-022-02814-6. Epub 2022 Apr 2. Mol Neurobiol. 2022. PMID: 35366734
-
Molecular Targets of Fatty Acid Ethanolamides in Asthma.Medicina (Kaunas). 2019 Apr 1;55(4):87. doi: 10.3390/medicina55040087. Medicina (Kaunas). 2019. PMID: 30939862 Free PMC article. Review.
References
-
- Ferriero DM. Neonatal brain injury. N Engl J Med. 2004;351:1985–1995 - PubMed
-
- Fernández-López D, Martínez-Orgado J, Casanova I, et al. . Immature rat brain slices exposed to oxygen-glucose deprivation as an in vitro model of neonatal hypoxic-ischemic encephalopathy. J Neurosci Methods. 2005;145:205–212 - PubMed
-
- Fernández-López D, Martínez-Orgado J, Nuñez E, et al. . Characterization of the neuroprotective effect of the cannabinoidagonist WIN-55212 in an in vitro model of hypoxic-ischemic brain damage in newborn rats. Pediatr Res. 2006;60:169–173 - PubMed
-
- Martinez-Orgado J, Fernandez-Lopez D, Moro MA, et al. . Nitric oxide synthase as a target for the prevention of hypoxic-ischemic newborn brain damage. Curr Enzym Inhib. 2006;2:219–229
-
- Bełtowski J, Wójcicka G, Mydlarczyk M, et al. . The effect of peroxisome proliferator-activated receptors alpha (PPARalpha) agonist, fenofibrate, on lipid peroxidation, total antioxidant capacity, and plasma paraoxonase 1 (PON 1) activity. J Physiol Pharmacol. 2002;53:463–475 - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
