Increase of PRPP enhances chemosensitivity of PRPS1 mutant acute lymphoblastic leukemia cells to 5-Fluorouracil
- PMID: 30255549
- PMCID: PMC6237573
- DOI: 10.1111/jcmm.13907
Increase of PRPP enhances chemosensitivity of PRPS1 mutant acute lymphoblastic leukemia cells to 5-Fluorouracil
Abstract
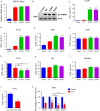
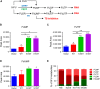
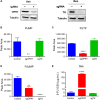
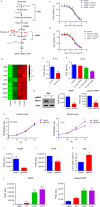
Relapse-specific mutations in phosphoribosyl pyrophosphate synthetase 1 (PRPS1), a rate-limiting purine biosynthesis enzyme, confer significant drug resistances to combination chemotherapy in acute lymphoblastic leukemia (ALL). It is of particular interest to identify drugs to overcome these resistances. In this study, we found that PRPS1 mutant ALL cells specifically showed more chemosensitivity to 5-Fluorouracil (5-FU) than control cells, attributed to increased apoptosis of PRPS1 mutant cells by 5-FU. Mechanistically, PRPS1 mutants increase the level of intracellular phosphoribosyl pyrophosphate (PRPP), which causes the apt conversion of 5-FU to FUMP and FUTP in Reh cells, to promote 5-FU-induced DNA damage and apoptosis. Our study not only provides mechanistic rationale for re-targeting drug resistant cells in ALL, but also implicates that ALL patients who harbor relapse-specific mutations of PRPS1 might benefit from 5-FU-based chemotherapy in clinical settings.
Keywords: 5-FU; PRPP; PRPS1; acute lymphoblastic leukemia; nucleotide metabolism.
© 2018 The Authors. Journal of Cellular and Molecular Medicine published by John Wiley & Sons Ltd and Foundation for Cellular and Molecular Medicine.
Figures

References
-
- Karran P, Attard N. Thiopurines in current medical practice: molecular mechanisms and contributions to therapy‐related cancer. Nat Rev Cancer. 2008;8:24‐36. - PubMed
-
- Raetz EA, Bhatla T. Where do we stand in the treatment of relapsed acute lymphoblastic leukemia?. Hematology Am Soc Hematol Educ Program. 2012;2012:129‐136. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

