Frequency and phenotype consequence of APOC3 rare variants in patients with very low triglyceride levels
- PMID: 30255797
- PMCID: PMC6156840
- DOI: 10.1186/s12920-018-0387-1
Frequency and phenotype consequence of APOC3 rare variants in patients with very low triglyceride levels
Abstract
Background: High levels of triglycerides (TG ≥200 mg/dL) are an emerging risk factor for cardiovascular disease. Conversely, very low levels of TG are associated with decreased risk for cardiovascular disease. Precision medicine aims to capitalize on recent findings that rare variants such as APOC3 R19X (rs76353203) are associated with risk of disease, but it is unclear how population-based associations can be best translated in clinical settings at the individual-patient level.
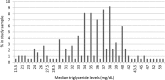
Methods: To explore the potential usefulness of screening for genetic predictors of cardiovascular disease, we surveyed BioVU, the Vanderbilt University Medical Center's biorepository linked to de-identified electronic health records (EHRs), for APOC3 19X mutations among adult European American patients (> 45 and > 55 years of age for men and women, respectively) with the lowest percentile of TG levels. The initial search identified 262 patients with the lowest TG levels in the biorepository; among these, 184 patients with sufficient DNA and the lowest TG levels were chosen for Illumina ExomeChip genotyping.
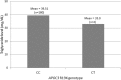
Results: A total of two patients were identified as heterozygotes of APOC3 R19X for a minor allele frequency (MAF) of 0.55% in this patient population. Both heterozygous patients had only a single mention of TG in the EHR (31 and 35 mg/dL, respectively), and one patient had evidence of previous cardiovascular disease.
Conclusions: In this patient population, we identified two patients who were carriers of the APOC3 19X null variant, but only one lacked evidence of disease in the EHR highlighting the challenges of inclusion of functional or previously associated genetic variation in clinical risk assessment.
Keywords: APOC3; Biobank; Electronic health records; Precision medicine; Triglycerides.
Conflict of interest statement
Ethics approval and consent to participate
The data in this study were de-identified in accordance with provisions of Title 45, Code of Federal Regulations, part 46 (45 CFR 46); therefore, this study was considered non-human subjects research by the Vanderbilt University Internal Review Board.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

