Kinetic mechanism of coupled binding in sodium-aspartate symporter GltPh
- PMID: 30255846
- PMCID: PMC6175574
- DOI: 10.7554/eLife.37291
Kinetic mechanism of coupled binding in sodium-aspartate symporter GltPh
Abstract
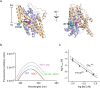
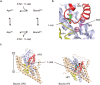
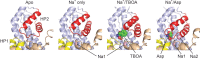
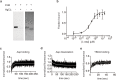
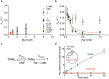
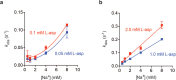
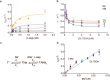
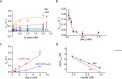
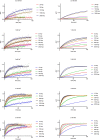
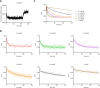
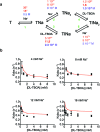
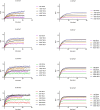
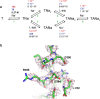
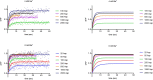
Many secondary active membrane transporters pump substrates against concentration gradients by coupling their uptake to symport of sodium ions. Symport requires the substrate and ions to be always transported together. Cooperative binding of the solutes is a key mechanism contributing to coupled transport in the sodium and aspartate symporter from Pyrococcus horikoshii GltPh. Here, we describe the kinetic mechanism of coupled binding for GltPh in the inward facing state. The first of the three coupled sodium ions, binds weakly and slowly, enabling the protein to accept the rest of the ions and the substrate. The last ion binds tightly, but is in rapid equilibrium with solution. Its release is required for the complex disassembly. Thus, the first ion serves to 'open the door' for the substrate, the last ion 'locks the door' once the substrate is in, and one ion contributes to both events.
Keywords: E. coli; binding kinetics; glutamate transporters; ion-coupled membrane transporters; molecular biophysics; structural biology.
© 2018, Oh et al.
Conflict of interest statement
SO No competing interests declared, OB Reviewing editor, eLife
Figures


References
-
- Adams PD, Afonine PV, Bunkóczi G, Chen VB, Davis IW, Echols N, Headd JJ, Hung LW, Kapral GJ, Grosse-Kunstleve RW, McCoy AJ, Moriarty NW, Oeffner R, Read RJ, Richardson DC, Richardson JS, Terwilliger TC, Zwart PH. PHENIX: a comprehensive Python-based system for macromolecular structure solution. Acta Crystallographica Section D Biological Crystallography. 2010;66:213–221. doi: 10.1107/S0907444909052925. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

