Biocatalytic synthesis of lactones and lactams
- PMID: 30256534
- PMCID: PMC6348383
- DOI: 10.1002/asia.201801180
Biocatalytic synthesis of lactones and lactams
Abstract
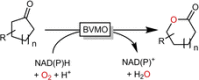
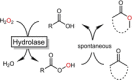
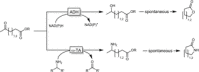
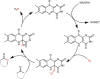
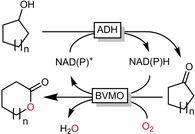
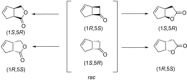
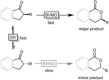
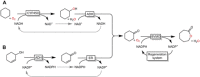
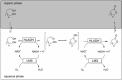
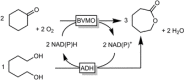
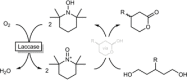
Cyclic esters and amides (lactones and lactams) are important active ingredients and polymer building blocks. In recent years, numerous biocatalytic methods for their preparation have been developed including enzymatic and chemoenzymatic Baeyer-Villiger oxidations, oxidative lactonisation of diols, and reductive lactonisation and lactamisation of ketoesters. The current state of the art of these methods is reviewed.
Keywords: Baeyer-Villiger oxidation; biocatalysis; lactams; lactones; oxidative Lactonisation.
© 2018 The Authors. Published by Wiley-VCH Verlag GmbH & Co. KGaA.
Figures
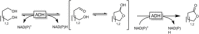
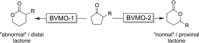
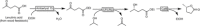
Similar articles
-
[Baeyer-Villiger monooxygenases in the biosynthesis of microbial secondary metabolites].Sheng Wu Gong Cheng Xue Bao. 2019 Mar 25;35(3):351-362. doi: 10.13345/j.cjb.180294. Sheng Wu Gong Cheng Xue Bao. 2019. PMID: 30912344 Review. Chinese.
-
C3 and C6 Modification-Specific OYE Biotransformations of Synthetic Carvones and Sequential BVMO Chemoenzymatic Synthesis of Chiral Caprolactones.Chemistry. 2019 Feb 26;25(12):2983-2988. doi: 10.1002/chem.201805219. Epub 2019 Jan 15. Chemistry. 2019. PMID: 30468546 Free PMC article.
-
Towards large-scale synthetic applications of Baeyer-Villiger monooxygenases.Trends Biotechnol. 2003 Jul;21(7):318-23. doi: 10.1016/S0167-7799(03)00144-6. Trends Biotechnol. 2003. PMID: 12837617 Review.
-
Recent developments in the application of Baeyer-Villiger monooxygenases as biocatalysts.Chembiochem. 2010 Nov 2;11(16):2208-31. doi: 10.1002/cbic.201000395. Chembiochem. 2010. PMID: 20936617 Review.
-
Discovery, application and protein engineering of Baeyer-Villiger monooxygenases for organic synthesis.Org Biomol Chem. 2012 Aug 21;10(31):6249-65. doi: 10.1039/c2ob25704a. Epub 2012 Jun 26. Org Biomol Chem. 2012. PMID: 22733152
Cited by
-
Biocatalysis: Enzymatic Synthesis for Industrial Applications.Angew Chem Int Ed Engl. 2021 Jan 4;60(1):88-119. doi: 10.1002/anie.202006648. Epub 2020 Aug 17. Angew Chem Int Ed Engl. 2021. PMID: 32558088 Free PMC article. Review.
-
Enzymatic Assembly of Diverse Lactone Structures: An Intramolecular C-H Functionalization Strategy.J Am Chem Soc. 2024 Jan 17;146(2):1580-1587. doi: 10.1021/jacs.3c11722. Epub 2024 Jan 2. J Am Chem Soc. 2024. PMID: 38166100 Free PMC article.
-
A Sustainable Green Enzymatic Method for Amide Bond Formation.Molecules. 2023 Jul 28;28(15):5706. doi: 10.3390/molecules28155706. Molecules. 2023. PMID: 37570676 Free PMC article.
-
Prospects of Using Biocatalysis for the Synthesis and Modification of Polymers.Molecules. 2021 May 7;26(9):2750. doi: 10.3390/molecules26092750. Molecules. 2021. PMID: 34067052 Free PMC article. Review.
-
Biological Activity of Selected Natural and Synthetic Terpenoid Lactones.Int J Mol Sci. 2021 May 10;22(9):5036. doi: 10.3390/ijms22095036. Int J Mol Sci. 2021. PMID: 34068609 Free PMC article. Review.
References
-
- Liu X., He L. N., Top. Curr. Chem. 2017, 375, 32. - PubMed
-
- None
-
- Ferroni F. M., Smit M. S., Opperman D. J., J. Mol. Catal. B 2014, 107, 47–54.
-
- Beneventi E., Ottolina G., Carrea G., Panzeri W., Fronza G., Lau P. C. K., J. Mol. Catal. B 2009, 58, 164–168.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

