A 6-Month, Prospective, Randomized Controlled Trial of Customized Adherence Enhancement Versus Bipolar-Specific Educational Control in Poorly Adherent Individuals With Bipolar Disorder
- PMID: 30256551
- PMCID: PMC6205506
- DOI: 10.4088/JCP.17m12036
A 6-Month, Prospective, Randomized Controlled Trial of Customized Adherence Enhancement Versus Bipolar-Specific Educational Control in Poorly Adherent Individuals With Bipolar Disorder
Abstract
Objective: Nonadherence in bipolar disorder (BD) ranges from 20% to 60%. Customized adherence enhancement (CAE) is a brief, BD-specific approach that targets individual adherence barriers. This prospective, 6-month, randomized controlled trial conducted from October 2012 to July 2017 compared CAE versus a rigorous BD-specific educational program (EDU) on adherence, symptoms, and functional outcomes in poorly adherent individuals.
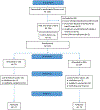
Methods: One hundred eighty-four participants with DSM-IV BD were randomized to CAE (n = 92) or EDU (n = 92). Primary outcome was adherence change measured by the Tablets Routine Questionnaire (TRQ) and BD symptoms measured by the Brief Psychiatric Rating Scale. Other outcomes were scores on the Global Assessment of Functioning, Montgomery-Asberg Depression Rating Scale, Young Mania Rating Scale, and Clinical Global Impressions Scale. Assessments were conducted at screening, baseline, 10 weeks, 14 weeks, and 6 months.
Results: The sample mean (SD) age was 47.40 (10.46) years; 68.5% were female, and 63.0% were African American. At screening, individuals missed a mean (SD) of 55.15% (28.22%) of prescribed BD drugs within the past week and 48.01% (28.46%) in the past month. Study attrition was < 20%. At 6 months, individuals in CAE had significantly improved past-week (P = .001) and past-month (P = .048) TRQ scores versus those in EDU. Past-week TRQ score improvement remained significant after adjustment for multiple comparisons. There were no treatment arm differences in BPRS scores or other symptoms, possibly related to low symptom baseline values. Baseline-to-6-month comparison showed significantly higher GAF scores (P = .036) for CAE versus EDU. Although both groups used more mental health services at 6 months compared to baseline, increase for CAE was significantly less than that for EDU (P = .046).
Conclusions: Whereas both CAE and EDU were associated with improved outcomes, CAE had additional positive effects on adherence, functioning, and mental health resource use compared to EDU.
Trial registration: ClinicalTrials.gov identifier: NCT00183495.
© Copyright 2018 Physicians Postgraduate Press, Inc.
Conflict of interest statement
Dr. Levin, Ms. Cassidy, Mr. Klein, Ms. Fuentes-Casiano, Dr. Blixen, and Ms. Aebi declare no conflicts of interest.
Figures
Similar articles
-
A 6-month, prospective, randomized controlled trial of customized adherence enhancement versus a bipolar-specific educational control in poorly adherent adolescents and young adults living with bipolar disorder.Bipolar Disord. 2024 Nov;26(7):696-707. doi: 10.1111/bdi.13489. Epub 2024 Sep 4. Bipolar Disord. 2024. PMID: 39231780 Clinical Trial.
-
Long-Acting Injectable Antipsychotic Medication Plus Customized Adherence Enhancement in Poor Adherence Patients With Bipolar Disorder.Prim Care Companion CNS Disord. 2021 Sep 16;23(5):20m02888. doi: 10.4088/PCC.20m02888. Prim Care Companion CNS Disord. 2021. PMID: 34534421 Clinical Trial.
-
Six-month outcomes of customized adherence enhancement (CAE) therapy in bipolar disorder.Bipolar Disord. 2012 May;14(3):291-300. doi: 10.1111/j.1399-5618.2012.01010.x. Bipolar Disord. 2012. PMID: 22548902 Free PMC article.
-
[Antipsychotics in bipolar disorders].Encephale. 2004 Sep-Oct;30(5):417-24. doi: 10.1016/s0013-7006(04)95456-5. Encephale. 2004. PMID: 15627046 Review. French.
-
Novel Augmentation Strategies in Major Depression.Dan Med J. 2017 Apr;64(4):B5338. Dan Med J. 2017. PMID: 28385173 Review.
Cited by
-
A 6-month, prospective, randomized controlled trial of customized adherence enhancement versus a bipolar-specific educational control in poorly adherent adolescents and young adults living with bipolar disorder.Bipolar Disord. 2024 Nov;26(7):696-707. doi: 10.1111/bdi.13489. Epub 2024 Sep 4. Bipolar Disord. 2024. PMID: 39231780 Clinical Trial.
-
Interventions to Improve Medication Adherence in Patients with Schizophrenia or Bipolar Disorders: A Systematic Review and Meta-Analysis.Int J Environ Res Public Health. 2021 Sep 28;18(19):10213. doi: 10.3390/ijerph181910213. Int J Environ Res Public Health. 2021. PMID: 34639510 Free PMC article.
-
Age-Related Differences in Medication Adherence, Symptoms, and Stigma in Poorly Adherent Adults With Bipolar Disorder.J Geriatr Psychiatry Neurol. 2020 Sep;33(5):250-255. doi: 10.1177/0891988719874116. Epub 2019 Sep 22. J Geriatr Psychiatry Neurol. 2020. PMID: 31542988 Free PMC article.
-
A randomized controlled trial of customized adherence enhancement (CAE-E): study protocol for a hybrid effectiveness-implementation project.Trials. 2022 Aug 4;23(1):634. doi: 10.1186/s13063-022-06517-0. Trials. 2022. PMID: 35927740 Free PMC article.
-
The Relationship Between Medication Attitudes and Medication Adherence Behavior in Adults With Bipolar Disorder.J Nerv Ment Dis. 2020 Feb;208(2):87-93. doi: 10.1097/NMD.0000000000001083. J Nerv Ment Dis. 2020. PMID: 31929465 Free PMC article.
References
-
- Lingam R, Scott J. Treatment non-adherence in affective disorders. Acta Psychiatr Scand. 2002;105(3):164–172. - PubMed
-
- Colom F, Vieta E, Martinez-Aran A, Reinares M, Benabarre A, Gasto C. Clinical factors associated with treatment noncompliance in euthymic bipolar patients. J Clin Psychiatry. 2000;61(8):549–555. - PubMed
-
- Sajatovic M, Valenstein M, Blow FC, Ganoczy D, Ignacio RV. Treatment adherence with antipsychotic medications in bipolar disorder. Bipolar Disord. 2006;8(3):232–241. - PubMed
-
- Licht RW, Vestergaard P, Rasmussen NA, Jepsen K, Brodersen A, Hansen PE. A lithium clinic for bipolar patients: 2-year outcome of the first 148 patients. Acta Psychiatr Scand. 2001;104(5):387–390. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous