Rab17 regulates apical delivery of hepatic transcytotic vesicles
- PMID: 30256711
- PMCID: PMC6249867
- DOI: 10.1091/mbc.E18-07-0433
Rab17 regulates apical delivery of hepatic transcytotic vesicles
Abstract
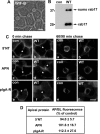
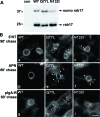
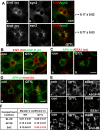
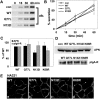
A major focus for our laboratory is identifying the molecules and mechanisms that regulate basolateral-to-apical transcytosis in polarized hepatocytes. Our most recent studies have focused on characterizing the biochemical and functional properties of the small rab17 GTPase. We determined that rab17 is a monosumoylated protein and that this modification likely mediates selective interactions with the apically located syntaxin 2. Using polarized hepatic WIF-B cells exogenously expressing wild-type, dominant active/guanosine triphosphate (GTP)-bound, dominant negative/guanosine diphosphate (GDP)-bound, or sumoylation-deficient/K68R rab17 proteins, we confirmed that rab17 regulates basolateral-to-apical transcytotic vesicle docking and fusion with the apical surface. We further confirmed that transcytosis is impaired from the subapical compartment to the apical surface and that GTP-bound and sumoylated rab17 are likely required for apical vesicle docking. Because expression of the GTP-bound rab17 led to impaired transcytosis, whereas wild type had no effect, we further propose that rab17 GTP hydrolysis is required for vesicle delivery. We also determined that transcytosis of three classes of newly synthesized apical residents showed similar responses to rab17 mutant expression, indicating that rab17 is a general component of the transcytotic machinery required for apically destined vesicle docking and fusion.
Figures




Similar articles
-
The GTP-bound and Sumoylated Form of the rab17 Small Molecular Weight GTPase Selectively Binds Syntaxin 2 in Polarized Hepatic WIF-B Cells.J Biol Chem. 2016 Apr 29;291(18):9721-32. doi: 10.1074/jbc.M116.723353. Epub 2016 Mar 8. J Biol Chem. 2016. PMID: 26957544 Free PMC article.
-
Rab17 localizes to recycling endosomes and regulates receptor-mediated transcytosis in epithelial cells.J Biol Chem. 1998 Jun 19;273(25):15734-41. doi: 10.1074/jbc.273.25.15734. J Biol Chem. 1998. PMID: 9624171
-
Rab17 regulates membrane trafficking through apical recycling endosomes in polarized epithelial cells.J Cell Biol. 1998 Mar 9;140(5):1039-53. doi: 10.1083/jcb.140.5.1039. J Cell Biol. 1998. PMID: 9490718 Free PMC article.
-
Mechanisms and functional features of polarized membrane traffic in epithelial and hepatic cells.Biochem J. 1998 Dec 1;336 ( Pt 2)(Pt 2):257-69. doi: 10.1042/bj3360257. Biochem J. 1998. PMID: 9820799 Free PMC article. Review.
-
GTP- and GDP-Dependent Rab27a Effectors in Pancreatic Beta-Cells.Biol Pharm Bull. 2015;38(5):663-8. doi: 10.1248/bpb.b14-00886. Biol Pharm Bull. 2015. PMID: 25947911 Review.
Cited by
-
MAL2 and rab17 selectively redistribute invadopodia proteins to laterally-induced protrusions in hepatocellular carcinoma cells.Mol Biol Cell. 2025 Mar 1;36(3):ar26. doi: 10.1091/mbc.E24-09-0400. Epub 2025 Jan 15. Mol Biol Cell. 2025. PMID: 39813085 Free PMC article.
-
Intercellular transfer of exosomal wild type EGFR triggers osimertinib resistance in non-small cell lung cancer.Mol Cancer. 2021 Jan 18;20(1):17. doi: 10.1186/s12943-021-01307-9. Mol Cancer. 2021. PMID: 33461557 Free PMC article.
-
SUMOylation of synaptic and synapse-associated proteins: An update.J Neurochem. 2021 Jan;156(2):145-161. doi: 10.1111/jnc.15103. Epub 2020 Jul 5. J Neurochem. 2021. PMID: 32538470 Free PMC article. Review.
-
High Resolution Proteomic Analysis of Subcellular Fractionated Boar Spermatozoa Provides Comprehensive Insights Into Perinuclear Theca-Residing Proteins.Front Cell Dev Biol. 2022 Feb 18;10:836208. doi: 10.3389/fcell.2022.836208. eCollection 2022. Front Cell Dev Biol. 2022. PMID: 35252197 Free PMC article.
-
Rho and Rab Family Small GTPases in the Regulation of Membrane Polarity in Epithelial Cells.Front Cell Dev Biol. 2022 Jul 4;10:948013. doi: 10.3389/fcell.2022.948013. eCollection 2022. Front Cell Dev Biol. 2022. PMID: 35859901 Free PMC article. Review.
References
-
- Aquea G, Bresky G, Lancellotti D, Madariaga JA, Zaffiri V, Urzua U, Haberle S, Bernal G. (2014). Increased expression of P2RY2, CD248 and EphB1 in gastric cancers from Chilean patients. Asian Pac J Cancer Prev , 1931–1936. - PubMed
-
- Barr VA, Hubbard AL. (1993). Newly synthesized hepatocyte plasma membrane proteins are transported in transcytotic vesicles in the bile duct-ligated rat. Gastroenterology , 554–571. - PubMed
-
- Bartles JR, Braiterman LT, Hubbard AL. (1985a). Biochemical characterization of domain-specific glycoproteins of the rat hepatocyte plasma membrane. J Biol Chem , 12792–12802. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

