Neuronal adaptation to sound statistics in the inferior colliculus of behaving macaques does not reduce the effectiveness of the masking noise
- PMID: 30256735
- PMCID: PMC6337033
- DOI: 10.1152/jn.00875.2017
Neuronal adaptation to sound statistics in the inferior colliculus of behaving macaques does not reduce the effectiveness of the masking noise
Abstract
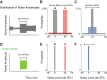
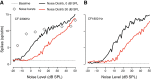
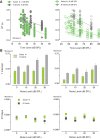
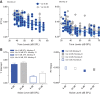
The detectability of target sounds embedded within noisy backgrounds is affected by the regularities that summarize acoustic sceneries. Previous studies suggested that the dynamic range of neurons in the inferior colliculus (IC) of anesthetized guinea pigs shifts toward the mean sound pressure level in irregular acoustic environments. Yet, it is unclear how this neuronal adaptation processes may influence the effectiveness of sounds as a masker, both behaviorally and in terms of neuronal encoding. To answer this question, we measured the neural response of IC neurons while macaque monkeys performed a Go/No-Go tone detection task. Macaques detected a 50-ms tone that was either simultaneously gated with a burst of noise or embedded within a continuous noise background, whose levels were randomly sampled (every 50 ms) from a probability distribution. The mean of the distribution matched the level of the gated burst of noise. Psychometric and IC neurometric thresholds to tones did not differ between the two masking conditions. However, the neuronal firing rate versus level function was significantly affected by the temporal characteristics of the noise masker. Simultaneously gated noise caused higher baseline responses and greater dynamic range compression compared with noise distribution. The slopes of psychometric and neurometric functions were significantly shallower for higher variance distributions, suggesting that neuronal sensitivity might change with the variability of the sound. Our results suggest that the adaptive response of IC neurons to sound regularities does not affect the effectiveness of the noise-masking signal, which remains invariant to surrounding noise amplitudes. NEW & NOTEWORTHY Auditory neurons adapt to the statistics of sound levels in the acoustic scene. However, it is still unclear to what extent such adaptation influences the effectiveness of the stimulus as a masker. Our study represents the first attempt to investigate how the adaptation to the statistics of masking stimuli may be related to the effectiveness of masking, and to the single-unit encoding of the midbrain auditory neurons in behaving animals.
Keywords: behaving macaques; dynamic range compression; inferior colliculus; neuronal adaptation; sound statistics.
Figures







Similar articles
-
Hearing in Complex Environments: Auditory Gain Control, Attention, and Hearing Loss.Front Neurosci. 2022 Feb 10;16:799787. doi: 10.3389/fnins.2022.799787. eCollection 2022. Front Neurosci. 2022. PMID: 35221899 Free PMC article. Review.
-
Foreground stimuli and task engagement enhance neuronal adaptation to background noise in the inferior colliculus of macaques.J Neurophysiol. 2020 Nov 1;124(5):1315-1326. doi: 10.1152/jn.00153.2020. Epub 2020 Sep 16. J Neurophysiol. 2020. PMID: 32937088 Free PMC article.
-
Spatial and temporal disparity in signals and maskers affects signal detection in non-human primates.Hear Res. 2017 Feb;344:1-12. doi: 10.1016/j.heares.2016.10.013. Epub 2016 Oct 19. Hear Res. 2017. PMID: 27770624 Free PMC article.
-
Neural correlates and mechanisms of spatial release from masking: single-unit and population responses in the inferior colliculus.J Neurophysiol. 2005 Aug;94(2):1180-98. doi: 10.1152/jn.01112.2004. Epub 2005 Apr 27. J Neurophysiol. 2005. PMID: 15857966 Free PMC article.
-
Hearing in noisy environments: noise invariance and contrast gain control.J Physiol. 2014 Aug 15;592(16):3371-81. doi: 10.1113/jphysiol.2014.274886. Epub 2014 Jun 6. J Physiol. 2014. PMID: 24907308 Free PMC article. Review.
Cited by
-
Three psychophysical metrics of auditory temporal integration in macaques.J Acoust Soc Am. 2021 Oct;150(4):3176. doi: 10.1121/10.0006658. J Acoust Soc Am. 2021. PMID: 34717465 Free PMC article.
-
Mechanisms of Tone-in-Noise Encoding in the Inferior Colliculus.J Neurosci. 2025 Jun 4;45(23):e1907242025. doi: 10.1523/JNEUROSCI.1907-24.2025. J Neurosci. 2025. PMID: 40335155
-
Hierarchical differences in the encoding of amplitude modulation in the subcortical auditory system of awake nonhuman primates.J Neurophysiol. 2024 Sep 1;132(3):1098-1114. doi: 10.1152/jn.00329.2024. Epub 2024 Aug 14. J Neurophysiol. 2024. PMID: 39140590 Free PMC article.
-
Listening in complex acoustic scenes.Curr Opin Physiol. 2020 Dec;18:63-72. doi: 10.1016/j.cophys.2020.09.001. Epub 2020 Sep 8. Curr Opin Physiol. 2020. PMID: 33479600 Free PMC article.
-
Hearing in Complex Environments: Auditory Gain Control, Attention, and Hearing Loss.Front Neurosci. 2022 Feb 10;16:799787. doi: 10.3389/fnins.2022.799787. eCollection 2022. Front Neurosci. 2022. PMID: 35221899 Free PMC article. Review.
References
-
- Bregman AS. Auditory Scene Analysis: The Perceptual Organization of Sound. Cambridge, MA: MIT Press, 1990.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

