Extrasynaptic α5GABAA receptors on proprioceptive afferents produce a tonic depolarization that modulates sodium channel function in the rat spinal cord
- PMID: 30256739
- PMCID: PMC6337028
- DOI: 10.1152/jn.00499.2018
Extrasynaptic α5GABAA receptors on proprioceptive afferents produce a tonic depolarization that modulates sodium channel function in the rat spinal cord
Abstract
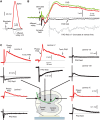
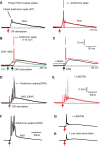
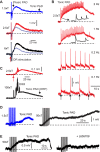
Activation of GABAA receptors on sensory axons produces a primary afferent depolarization (PAD) that modulates sensory transmission in the spinal cord. While axoaxonic synaptic contacts of GABAergic interneurons onto afferent terminals have been extensively studied, less is known about the function of extrasynaptic GABA receptors on afferents. Thus, we examined extrasynaptic α5GABAA receptors on low-threshold proprioceptive (group Ia) and cutaneous afferents. Afferents were impaled with intracellular electrodes and filled with neurobiotin in the sacrocaudal spinal cord of rats. Confocal microscopy was used to reconstruct the afferents and locate immunolabelled α5GABAA receptors. In all afferents α5GABAA receptors were found throughout the extensive central axon arbors. They were most densely located at branch points near sodium channel nodes, including in the dorsal horn. Unexpectedly, proprioceptive afferent terminals on motoneurons had a relative lack of α5GABAA receptors. When recording intracellularly from these afferents, blocking α5GABAA receptors (with L655708, gabazine, or bicuculline) hyperpolarized the afferents, as did blocking neuronal activity with tetrodotoxin, indicating a tonic GABA tone and tonic PAD. This tonic PAD was increased by repeatedly stimulating the dorsal root at low rates and remained elevated for many seconds after the stimulation. It is puzzling that tonic PAD arises from α5GABAA receptors located far from the afferent terminal where they can have relatively little effect on terminal presynaptic inhibition. However, consistent with the nodal location of α5GABAA receptors, we find tonic PAD helps produce sodium spikes that propagate antidromically out the dorsal roots, and we suggest that it may well be involved in assisting spike transmission in general. NEW & NOTEWORTHY GABAergic neurons are well known to form synaptic contacts on proprioceptive afferent terminals innervating motoneurons and to cause presynaptic inhibition. However, the particular GABA receptors involved are unknown. Here, we examined the distribution of extrasynaptic α5GABAA receptors on proprioceptive Ia afferents. Unexpectedly, these receptors were found preferentially near nodal sodium channels throughout the afferent and were largely absent from afferent terminals. These receptors produced a tonic afferent depolarization that modulated sodium spikes, consistent with their location.
Keywords: GABA; antidromic action potential; branch point failure; dorsal root; dorsal root reflex; extrasynaptic; intracellular recording; primary afferent depolarization; spinal cord.
Figures








Similar articles
-
Facilitation of sensory transmission to motoneurons during cortical or sensory-evoked primary afferent depolarization (PAD) in humans.J Physiol. 2023 May;601(10):1897-1924. doi: 10.1113/JP284275. Epub 2023 Mar 27. J Physiol. 2023. PMID: 36916205 Free PMC article.
-
α(5)GABA(A) receptors mediate primary afferent fiber tonic excitability in the turtle spinal cord.J Neurophysiol. 2013 Nov;110(9):2175-84. doi: 10.1152/jn.00330.2013. Epub 2013 Aug 21. J Neurophysiol. 2013. PMID: 23966669
-
Bicuculline-sensitive primary afferent depolarization remains after greatly restricting synaptic transmission in the mammalian spinal cord.J Neurosci. 2010 Apr 14;30(15):5283-8. doi: 10.1523/JNEUROSCI.3873-09.2010. J Neurosci. 2010. PMID: 20392950 Free PMC article.
-
Presynaptic selection of afferent inflow in the spinal cord.J Physiol Paris. 1999 Sep-Oct;93(4):329-47. doi: 10.1016/s0928-4257(00)80061-3. J Physiol Paris. 1999. PMID: 10574122 Review.
-
Antidromic discharges of dorsal root afferents in the neonatal rat.J Physiol Paris. 1999 Sep-Oct;93(4):359-67. doi: 10.1016/s0928-4257(00)80063-7. J Physiol Paris. 1999. PMID: 10574124 Review.
Cited by
-
Modulation of Sensory Input to the Spinal Cord by Peripheral Afferent Fibres via GABAergic Astrocytes.Eur J Neurosci. 2025 Mar;61(6):e70057. doi: 10.1111/ejn.70057. Eur J Neurosci. 2025. PMID: 40123195 Free PMC article.
-
Locomotor-related V3 interneurons initiate and coordinate muscles spasms after spinal cord injury.J Neurophysiol. 2019 Apr 1;121(4):1352-1367. doi: 10.1152/jn.00776.2018. Epub 2019 Jan 9. J Neurophysiol. 2019. PMID: 30625014 Free PMC article.
-
Post-activation depression from primary afferent depolarization (PAD) produces extensor H-reflex suppression following flexor afferent conditioning.J Physiol. 2023 May;601(10):1925-1956. doi: 10.1113/JP283706. Epub 2023 Apr 9. J Physiol. 2023. PMID: 36928599 Free PMC article.
-
Motor Unit Discharge Patterns in Response to Focal Tendon Vibration of the Lower Limb in Cats and Humans.Front Integr Neurosci. 2022 Apr 26;16:836757. doi: 10.3389/fnint.2022.836757. eCollection 2022. Front Integr Neurosci. 2022. PMID: 35558155 Free PMC article.
-
The structure of sensory afferent compartments in health and disease.J Anat. 2022 Nov;241(5):1186-1210. doi: 10.1111/joa.13544. Epub 2021 Sep 15. J Anat. 2022. PMID: 34528255 Free PMC article. Review.
References
-
- Alvarez FJ. Anatomical basis for presynaptic inhibition of primary sensory fibers. In: Presynaptic Inhibition and Neuron Control, edited by Rudmon P, Romo R, Mendell LM,. New York: Oxford University Press, 1998, p. 13–41.
-
- Amrutkar DV, Ploug KB, Hay-Schmidt A, Porreca F, Olesen J, Jansen-Olesen I. mRNA expression of 5-hydroxytryptamine 1B, 1D, and 1F receptors and their role in controlling the release of calcitonin gene-related peptide in the rat trigeminovascular system. Pain 153: 830–838, 2012. doi:10.1016/j.pain.2012.01.005. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

