A novel device for real-time measurement and manipulation of licking behavior in head-fixed mice
- PMID: 30256741
- PMCID: PMC6442917
- DOI: 10.1152/jn.00500.2018
A novel device for real-time measurement and manipulation of licking behavior in head-fixed mice
Abstract
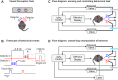
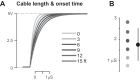
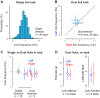
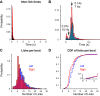
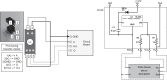
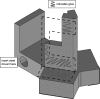
The mouse has become an influential model system for investigating the mammalian nervous system. Technologies in mice enable recording and manipulation of neural circuits during tasks where they respond to sensory stimuli by licking for liquid rewards. Precise monitoring of licking during these tasks provides an accessible metric of sensory-motor processing, particularly when combined with simultaneous neural recordings. There are several challenges in designing and implementing lick detectors during head-fixed neurophysiological experiments in mice. First, mice are small, and licking behaviors are easily perturbed or biased by large sensors. Second, neural recordings during licking are highly sensitive to electrical contact artifacts. Third, submillisecond lick detection latencies are required to generate control signals that manipulate neural activity at appropriate time scales. Here we designed, characterized, and implemented a contactless dual-port device that precisely measures directional licking in head-fixed mice performing visual behavior. We first determined the optimal characteristics of our detector through design iteration and then quantified device performance under ideal conditions. We then tested performance during head-fixed mouse behavior with simultaneous neural recordings in vivo. We finally demonstrate our device's ability to detect directional licks and generate appropriate control signals in real time to rapidly suppress licking behavior via closed-loop inhibition of neural activity. Our dual-port detector is cost effective and easily replicable, and it should enable a wide variety of applications probing the neural circuit basis of sensory perception, motor action, and learning in normal and transgenic mouse models. NEW & NOTEWORTHY Mice readily learn tasks in which they respond to sensory cues by licking for liquid rewards; tasks that involve multiple licking responses allow study of neural circuits underlying decision making and sensory-motor integration. Here we design, characterize, and implement a novel dual-port lick detector that precisely measures directional licking in head-fixed mice performing visual behavior, enabling simultaneous neural recording and closed-loop manipulation of licking.
Keywords: closed-loop optogenetics; head-fixed behavior; licking; mouse; silicon probe.
Figures









Similar articles
-
Procedures for behavioral experiments in head-fixed mice.PLoS One. 2014 Feb 10;9(2):e88678. doi: 10.1371/journal.pone.0088678. eCollection 2014. PLoS One. 2014. PMID: 24520413 Free PMC article.
-
A novel method for detecting licking behavior during recording of electrophysiological signals from the brain.J Neurosci Methods. 2001 Apr 30;106(2):139-46. doi: 10.1016/s0165-0270(01)00341-7. J Neurosci Methods. 2001. PMID: 11325433
-
Natural whisker-guided behavior by head-fixed mice in tactile virtual reality.J Neurosci. 2014 Jul 16;34(29):9537-50. doi: 10.1523/JNEUROSCI.0712-14.2014. J Neurosci. 2014. PMID: 25031397 Free PMC article.
-
Licking behavior in the rat: measurement and situational control of licking frequency.Neurosci Biobehav Rev. 1998 Oct;22(6):751-60. doi: 10.1016/s0149-7634(98)00003-7. Neurosci Biobehav Rev. 1998. PMID: 9809310 Review.
-
Neocortical dynamics during whisker-based sensory discrimination in head-restrained mice.Neuroscience. 2018 Jan 1;368:57-69. doi: 10.1016/j.neuroscience.2017.09.003. Epub 2017 Sep 14. Neuroscience. 2018. PMID: 28919043 Free PMC article. Review.
Cited by
-
Optimizing intact skull intrinsic signal imaging for subsequent targeted electrophysiology across mouse visual cortex.Sci Rep. 2022 Feb 8;12(1):2063. doi: 10.1038/s41598-022-05932-2. Sci Rep. 2022. PMID: 35136111 Free PMC article.
-
Diminished Cortical Excitation and Elevated Inhibition During Perceptual Impairments in a Mouse Model of Autism.Cereb Cortex. 2021 Jun 10;31(7):3462-3474. doi: 10.1093/cercor/bhab025. Cereb Cortex. 2021. PMID: 33677512 Free PMC article.
-
Medial entorhinal-hippocampal desynchronization parallels the emergence of memory impairment in a mouse model of Alzheimer's disease pathology.bioRxiv [Preprint]. 2025 Jan 16:2025.01.15.633171. doi: 10.1101/2025.01.15.633171. bioRxiv. 2025. PMID: 39868201 Free PMC article. Preprint.
-
A paradigm for ethanol consumption in head-fixed mice during prefrontal cortical two-photon calcium imaging.bioRxiv [Preprint]. 2023 Jul 22:2023.07.20.549846. doi: 10.1101/2023.07.20.549846. bioRxiv. 2023. Update in: Neuropharmacology. 2024 Mar 1;245:109800. doi: 10.1016/j.neuropharm.2023.109800. PMID: 37503061 Free PMC article. Updated. Preprint.
-
FreiBox: A Versatile Open-Source Behavioral Setup for Investigating the Neuronal Correlates of Behavioral Flexibility via 1-Photon Imaging in Freely Moving Mice.eNeuro. 2023 Apr 27;10(4):ENEURO.0469-22.2023. doi: 10.1523/ENEURO.0469-22.2023. Print 2023 Apr. eNeuro. 2023. PMID: 37105720 Free PMC article.
References
-
- Burgess CP, Lak A, Steinmetz NA, Zatka-Haas P, Bai Reddy C, Jacobs EAK, Linden JF, Paton JJ, Ranson A, Schröder S, Soares S, Wells MJ, Wool LE, Harris KD, Carandini M. High-yield methods for accurate two-alternative visual psychophysics in head-fixed mice. Cell Reports 20: 2513–2524, 2017. doi:10.1016/j.celrep.2017.08.047. - DOI - PMC - PubMed
-
- Guo ZV, Hires SA, Li N, O’Connor DH, Komiyama T, Ophir E, Huber D, Bonardi C, Morandell K, Gutnisky D, Peron S, Xu NL, Cox J, Svoboda K. Procedures for behavioral experiments in head-fixed mice. PLoS One 9: e88678, 2014b. [Erratum in PLOS One 9: e101397; 10.1371/journal.pone.0101397] 10.1371/journal.pone.0088678. - DOI - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

