Mechanisms of acquired resistance to rapalogs in metastatic renal cell carcinoma
- PMID: 30256787
- PMCID: PMC6181431
- DOI: 10.1371/journal.pgen.1007679
Mechanisms of acquired resistance to rapalogs in metastatic renal cell carcinoma
Abstract
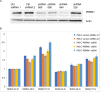
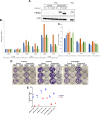
The mechanistic target of rapamycin (mTOR) is an established therapeutic target in renal cell carcinoma (RCC). Mechanisms of secondary resistance to rapalog therapy in RCC have not been studied previously. We identified six patients with metastatic RCC who initially responded to mTOR inhibitor therapy and then progressed, and had pre-treatment and post-treatment tumor samples available for analysis. We performed deep whole exome sequencing on the paired tumor samples and a blood sample. Sequence data was analyzed using Mutect, CapSeg, Absolute, and Phylogic to identify mutations, copy number changes, and their changes over time. We also performed in vitro functional assays on PBRM1 in RCC cell lines. Five patients had clear cell and one had chromophobe RCC. 434 somatic mutations in 416 genes were identified in the 12 tumor samples. 201 (46%) of mutations were clonal in both samples while 129 (30%) were acquired in the post-treatment samples. Tumor heterogeneity or sampling issues are likely to account for some mutations that were acquired in the post-treatment samples. Three samples had mutations in TSC1; one in PTEN; and none in MTOR. PBRM1 was the only gene in which mutations were acquired in more than one post-treatment sample. We examined the effect of PBRM1 loss in multiple RCC cell lines, and could not identify any effect on rapalog sensitivity in in vitro culture assays. We conclude that mTOR pathway gene mutations did not contribute to rapalog resistance development in these six patients with advanced RCC. Furthermore, mechanisms of resistance to rapalogs in RCC remain unclear and our results suggest that PBRM1 loss may contribute to sensitivity through complex transcriptional effects.
Conflict of interest statement
I have read the journal's policy and the authors of this manuscript have the following competing interests: DFM is a consultant/advisory board member for Novartis and Pfizer. DJK is a consultant/advisory board member for Novartis and leads a Novartis-sponsored clinical trial. TKC received research funds from Pfizer, GSK, Novartis, BMS, Merck, Exelixis, Roche, Astra Zeneca, Tracon and is a consultant/advisory board member for Pfizer, GSK, Novartis, Roche, Merck and Bayer. NA is a consultant/advisory board member for Pfizer, Novartis, Merck, Genentech, Eisai, Exelixis, Clovis, EMD Serono, BMS, AstraZeneca, and Astellas. SS received research funds from BMS, Exelixis, AstraZeneca and is a consultant/advisory board member for AstraZeneca and Merck.
Figures
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

