Regenerative glutamate release in the hippocampus of Rett syndrome model mice
- PMID: 30256804
- PMCID: PMC6157837
- DOI: 10.1371/journal.pone.0202802
Regenerative glutamate release in the hippocampus of Rett syndrome model mice
Abstract
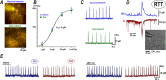
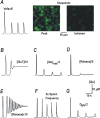
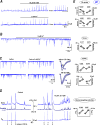
Excess glutamate during intense neuronal activity is not instantly cleared and may accumulate in the extracellular space. This has various long-term consequences such as ectopic signaling, modulation of synaptic efficacy and excitotoxicity; the latter implicated in various neurodevelopmental and neurodegenerative diseases. In this study, the quantitative imaging of glutamate homeostasis of hippocampal slices from methyl-CpG binding protein 2 knock-out (Mecp2-/y) mice, a model of Rett syndrome (RTT), revealed unusual repetitive glutamate transients. They appeared in phase with bursts of action potentials in the CA1 neurons. Both glutamate transients and bursting activity were suppressed by the blockade of sodium, AMPA and voltage-gated calcium channels (T- and R-type), and enhanced after the inhibition of HCN channels. HCN and calcium channels in RTT and wild-type (WT) CA1 neurons displayed different voltage-dependencies and kinetics. Both channels modulated postsynaptic integration and modified the pattern of glutamate spikes in the RTT hippocampus. Spontaneous glutamate transients were much less abundant in the WT preparations, and, when observed, had smaller amplitude and frequency. The basal ambient glutamate levels in RTT were higher and transient glutamate increases (spontaneous and evoked by stimulation of Schaffer collaterals) decayed slower. Both features indicate less efficient glutamate uptake in RTT. To explain the generation of repetitive glutamate spikes, we designed a novel model of glutamate-induced glutamate release. The simulations correctly predicted the patterns of spontaneous glutamate spikes observed under different experimental conditions. We propose that pervasive spontaneous glutamate release is a hallmark of Mecp2-/y hippocampus, stemming from and modulating the hyperexcitability of neurons.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures





References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

