A neoepitope derived from a novel human germline APC gene mutation in familial adenomatous polyposis shows selective immunogenicity
- PMID: 30256815
- PMCID: PMC6157866
- DOI: 10.1371/journal.pone.0203845
A neoepitope derived from a novel human germline APC gene mutation in familial adenomatous polyposis shows selective immunogenicity
Abstract
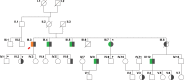
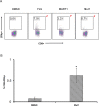
Familial adenomatous polyposis (FAP) is an inherited condition arising from genetic defects in the Adenomatous polyposis coli (APC) gene. Carriers with mutations in the APC gene develop polyps in the colon and rectum which if not managed, transition into colon cancer. In this study, we identified a novel germline mutation in the APC gene in members of an FAP-affected (Familial adenomatous polyposis) family. This unique heterozygous variant (c.735_736insT; p.Ser246PhefsTer6) was identified in ten out of twenty six family members, ranging in age from 6 to 60 years. Polyps were detected in six of the ten individuals (35-60 years) carrying this mutation. The remaining four members (6-23 years) remain polyp free. A significant fraction of FAP affected individuals eventually develop colon cancer and therapeutic interventions to prevent cancer progression remain elusive. To address this issue, we sought to determine if peptides derived from the novel APC mutation could induce a cytotoxic T cell response, thereby qualifying them as vaccine candidates. Peptides harboring the variant amino acids were first interrogated in silico for their immunogenicity using a proprietary neoepitope prioritization pipeline, OncoPeptVAC. A single 9-mer peptide was predicted to be immunogenic. Remarkably, CD8+ T cells isolated from either an FAP+/ APCmut individual, or from a FAP-/ APCmut individual, failed to respond to the peptide, whereas those from either an unaffected family member (FAP-/ APCwt) or from healthy unrelated donors, showed a robust response, suggesting that CD8+ T cells from individuals carrying this germline APC mutation have been tolerized to the mutation. Furthermore, experimental testing of six additional reported APC gene mutation-derived peptides revealed one of the six to be immunogenic. While not all APC mutant peptides are inmmunogenic, a few qualify as vaccine candidates offering novel treatment opportunities to patients with somatic APC gene mutations to delay/treat colorectal cancer.
Conflict of interest statement
The following authors are employed by MedGenome Labs Pvt. Ltd., MedGenome Inc: SMajumder, JE, KC, PS, AKM, BM, JKD, SMurugan, LM, RS, VR, PC, RG, AC and AK-G, and have no additional commercial affiliation relating to employment, consultancy, patents, products in development, or marketed products. Our commercial affiliation with MedGenome Labs does not alter adherence to all PLOS ONE policies on sharing data and materials. We declare that this does not alter our adherence to PLOS ONE policies on sharing data and materials.
Figures


Similar articles
-
Targeted next-generation sequencing approach for molecular genetic diagnosis of hereditary colorectal cancer: Identification of a novel single nucleotide germline insertion in adenomatous polyposis coli gene causes familial adenomatous polyposis.Mol Genet Genomic Med. 2019 Jan;7(1):e00505. doi: 10.1002/mgg3.505. Epub 2018 Dec 6. Mol Genet Genomic Med. 2019. PMID: 30523670 Free PMC article.
-
A novel pathogenic splice acceptor site germline mutation in intron 14 of the APC gene in a Chinese family with familial adenomatous polyposis.Oncotarget. 2017 Mar 28;8(13):21327-21335. doi: 10.18632/oncotarget.15570. Oncotarget. 2017. PMID: 28423518 Free PMC article.
-
Heterozygous APC germline mutations impart predisposition to colorectal cancer.Sci Rep. 2021 Mar 4;11(1):5113. doi: 10.1038/s41598-021-84564-4. Sci Rep. 2021. PMID: 33664379 Free PMC article.
-
The genetics of familial adenomatous polyposis (FAP) and MutYH-associated polyposis (MAP).Acta Gastroenterol Belg. 2011 Sep;74(3):421-6. Acta Gastroenterol Belg. 2011. PMID: 22103048 Review.
-
APC germline mutations in individuals being evaluated for familial adenomatous polyposis: a review of the Mayo Clinic experience with 1591 consecutive tests.J Mol Diagn. 2013 Jan;15(1):31-43. doi: 10.1016/j.jmoldx.2012.07.005. Epub 2012 Nov 14. J Mol Diagn. 2013. PMID: 23159591 Review.
Cited by
-
The Adaptive Immune Landscape of the Colorectal Adenoma-Carcinoma Sequence.Int J Mol Sci. 2021 Sep 10;22(18):9791. doi: 10.3390/ijms22189791. Int J Mol Sci. 2021. PMID: 34575971 Free PMC article.
-
Association of Pathogenic Missense and Nonsense Mutations in Mitochondrial COII Gene with Familial Adenomatous Polyposis (FAP).Int J Mol Cell Med. 2020 Fall;9(4):255-265. doi: 10.22088/IJMCM.BUMS.9.4.255. Epub 2021 Jan 27. Int J Mol Cell Med. 2020. PMID: 33688483 Free PMC article.
-
Vaccines for cancer interception in familial adenomatous polyposis.Front Immunol. 2025 Jan 29;16:1525157. doi: 10.3389/fimmu.2025.1525157. eCollection 2025. Front Immunol. 2025. PMID: 39944699 Free PMC article. Review.
-
Identifying and Targeting Human Tumor Antigens for T Cell-Based Immunotherapy of Solid Tumors.Cancer Cell. 2020 Oct 12;38(4):454-472. doi: 10.1016/j.ccell.2020.07.013. Epub 2020 Aug 20. Cancer Cell. 2020. PMID: 32822573 Free PMC article. Review.
References
-
- Nandakumar G, Morgan JA, Silverberg D, Steinhagen RM. Familial polyposis coli: Clinical manifestations, evaluation, management and treatment. Mt Sinai J Med. 2004;71: 384–391. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

