Ethanol and unsaturated dietary fat induce unique patterns of hepatic ω-6 and ω-3 PUFA oxylipins in a mouse model of alcoholic liver disease
- PMID: 30256818
- PMCID: PMC6157879
- DOI: 10.1371/journal.pone.0204119
Ethanol and unsaturated dietary fat induce unique patterns of hepatic ω-6 and ω-3 PUFA oxylipins in a mouse model of alcoholic liver disease
Abstract
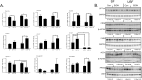
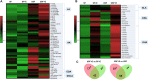
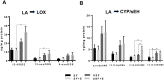
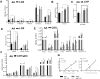
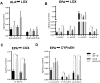
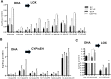
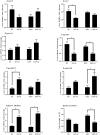
Alcoholic liver disease (ALD), a significant health problem, progresses through the course of several pathologies including steatosis, steatohepatitis, fibrosis, and cirrhosis. There are no effective FDA-approved medications to prevent or treat any stages of ALD, and the mechanisms involved in ALD pathogenesis are not well understood. Bioactive lipid metabolites play a crucial role in numerous pathological conditions, as well as in the induction and resolution of inflammation. Herein, a hepatic lipidomic analysis was performed on a mouse model of ALD with the objective of identifying novel metabolic pathways and lipid mediators associated with alcoholic steatohepatitis, which might be potential novel biomarkers and therapeutic targets for the disease. We found that ethanol and dietary unsaturated, but not saturated, fat caused elevated plasma ALT levels, hepatic steatosis and inflammation. These pathologies were associated with increased levels of bioactive lipid metabolites generally involved in pro-inflammatory responses, including 13-hydroxy-octadecadienoic acid, 9,10- and 12,13-dihydroxy-octadecenoic acids, 5-, 8-, 9-, 11-, 15-hydroxy-eicosatetraenoic acids, and 8,9- and 11,12-dihydroxy-eicosatrienoic acids, in parallel with an increase in pro-resolving mediators, such as lipoxin A4, 18-hydroxy-eicosapentaenoic acid, and 10S,17S-dihydroxy-docosahexaenoic acid. Elucidation of alterations in these lipid metabolites may shed new light into the molecular mechanisms underlying ALD development/progression, and be potential novel therapeutic targets.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures



Similar articles
-
n-3 Polyunsaturated fatty acids for the management of alcoholic liver disease: A critical review.Crit Rev Food Sci Nutr. 2019;59(sup1):S116-S129. doi: 10.1080/10408398.2018.1544542. Epub 2018 Dec 22. Crit Rev Food Sci Nutr. 2019. PMID: 30580553 Review.
-
Dietary Linoleic Acid and Its Oxidized Metabolites Exacerbate Liver Injury Caused by Ethanol via Induction of Hepatic Proinflammatory Response in Mice.Am J Pathol. 2017 Oct;187(10):2232-2245. doi: 10.1016/j.ajpath.2017.06.008. Am J Pathol. 2017. PMID: 28923202 Free PMC article.
-
Omega-6 and omega-3 oxylipins are implicated in soybean oil-induced obesity in mice.Sci Rep. 2017 Oct 2;7(1):12488. doi: 10.1038/s41598-017-12624-9. Sci Rep. 2017. PMID: 28970503 Free PMC article.
-
Saturated and Unsaturated Dietary Fats Differentially Modulate Ethanol-Induced Changes in Gut Microbiome and Metabolome in a Mouse Model of Alcoholic Liver Disease.Am J Pathol. 2016 Apr;186(4):765-76. doi: 10.1016/j.ajpath.2015.11.017. Am J Pathol. 2016. PMID: 27012191 Free PMC article.
-
Binge ethanol and liver: new molecular developments.Alcohol Clin Exp Res. 2013 Apr;37(4):550-7. doi: 10.1111/acer.12011. Epub 2013 Jan 24. Alcohol Clin Exp Res. 2013. PMID: 23347137 Free PMC article. Review.
Cited by
-
Fat-1 Transgenic Mice With Augmented n3-Polyunsaturated Fatty Acids Are Protected From Liver Injury Caused by Acute-On-Chronic Ethanol Administration.Front Pharmacol. 2021 Aug 31;12:711590. doi: 10.3389/fphar.2021.711590. eCollection 2021. Front Pharmacol. 2021. PMID: 34531743 Free PMC article.
-
Hepatic oxylipin profiles in mouse models of Wilson disease: New insights into early hepatic manifestations.Biochim Biophys Acta Mol Cell Biol Lipids. 2024 Mar;1869(2):159446. doi: 10.1016/j.bbalip.2023.159446. Epub 2023 Dec 9. Biochim Biophys Acta Mol Cell Biol Lipids. 2024. PMID: 38072238 Free PMC article.
-
Decreased ω-6:ω-3 PUFA ratio attenuates ethanol-induced alterations in intestinal homeostasis, microbiota, and liver injury.J Lipid Res. 2019 Dec;60(12):2034-2049. doi: 10.1194/jlr.RA119000200. Epub 2019 Oct 4. J Lipid Res. 2019. PMID: 31586017 Free PMC article.
-
Alcoholic hepatitis and metabolic disturbance in female mice: a more tractable model than Nrf2-/- animals.Dis Model Mech. 2020 Dec 29;13(12):dmm046383. doi: 10.1242/dmm.046383. Dis Model Mech. 2020. PMID: 33067186 Free PMC article.
-
Integrating comprehensive two-dimensional gas chromatography mass spectrometry and parallel two-dimensional liquid chromatography mass spectrometry for untargeted metabolomics.Analyst. 2019 Jul 21;144(14):4331-4341. doi: 10.1039/c9an00560a. Epub 2019 Jun 13. Analyst. 2019. PMID: 31192319 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

