Tunable activation of therapeutic platelet-rich plasma by pulse electric field: Differential effects on clot formation, growth factor release, and platelet morphology
- PMID: 30256831
- PMCID: PMC6157860
- DOI: 10.1371/journal.pone.0203557
Tunable activation of therapeutic platelet-rich plasma by pulse electric field: Differential effects on clot formation, growth factor release, and platelet morphology
Abstract
Background: Activation of platelet-rich plasma (PRP) by pulse electric field (PEF) releases growth factors which promote wound healing (e.g., PDGF, VEGF for granulation, EGF for epithelialization).
Aims: To determine after PEF activation of therapeutic PRP: 1) platelet gel strength; 2) profile of released growth factors; 3) alpha- and T-granule release; 4) platelet morphology.
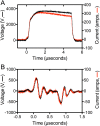
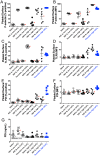
Methods: Concentrated normal donor PRP was activated by 5 μsec (long) monopolar pulse, ~4000 V/cm (PEF A) or 150 nsec (short) bipolar pulse, ~3000 V/cm (PEF B) in the presence of 2.5 mM (low) or 20 mM (high) added CaCl2. Clot formation was evaluated by thromboelastography (TEG). Surface exposure of alpha granule (P-selectin) and T-granule (TLR9 and protein disulfide isomerase [PDI]) markers were assessed by flow cytometry. Factors in supernatants of activated PRP were measured by ELISA. Platelet morphology was evaluated by transmission electron microscopy (TEM).
Results: Time to initial clot formation was shorter with thrombin (<1 min) than with PEF A and B (4.4-8.7 min) but clot strength (elastic modulus, derived from TEG maximum amplitude) was greater with PEF B than with either thrombin or PEF A (p<0.05). Supernatants of PRP activated with PEF A had higher EGF levels than supernatants from all other conditions. In contrast, levels of PF4, PDGF, and VEGF in supernatants were not significantly different after PEF A, PEF B, and thrombin activation. T-granule markers (TLR9 and PDI) were higher after thrombin than after PEF A or B with low or high CaCl2. By TEM, platelets in PEF-treated samples retained a subset of granules suggesting regulated granule release.
Conclusion: Pulse length and polarity can be modulated to produce therapeutic platelet gels as strong or stronger than those produced by thrombin, and this is tunable to produce growth factor profiles enhanced in specific factors important for different stages of wound healing.
Conflict of interest statement
I have read the journal’s policy and the authors of this manuscript have the following competing interests: A.L. Frelinger and A.D. Michelson received research support from GE Healthcare. A. Caiafa, and V. Neculaes are employees of GE Healthcare. A.S. Torres is a former employee of GE Healthcare. This does not alter the authors’ adherence to PLOS ONE policies on sharing data and materials. The remaining authors declare no competing interests.
Figures





References
-
- Klement GL, Shai S, Varon D (2013) The role of platelets in angiogenesis In: Michelson AD, editor. Platelets. 3rd ed San Diego: Elsevier/Academic Press; pp. 487–502.
-
- Driver VR, Hanft J, Fylling CP, Beriou JM, Autologel Diabetic Foot Ulcer Study Group (2006) A prospective, randomized, controlled trial of autologous platelet-rich plasma gel for the treatment of diabetic foot ulcers. Ostomy Wound Management 52: 68–74. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

